3622
Discrimination between High-Grade Gliomas and Solitary Brain Metastases in Peritumoral Brain Zone: Qualitative Analysis Using Relaxation Maps1Ningxia Medical University, Yinchuan, China, 2General Hospital of Ningxia Medical University, Yinchuan, China, 3GE Healthcare, MR Research, Beijing, China, 4GE Healthcare, MR Enhancement Application, Beijing, China
Synopsis
This work sought to identify a new non-invasive means to differentiate high-grade gliomas (HGGs) from solitary brain metastases (SBMs). It was concluded that the T1native, T2native, PDnative, and ΔT1ratio values were measured in peritumoral brain zone from synthetic MRI can be used as quantitative imaging biomarkers for distinguishing between HGGs and SBMs. The ΔT1ratio values have higher discrimination abilities compared with other parameters, which worth further study.
Introduction
High-grade gliomas (HGGs), the most malignant subtype of neuroepithelial tumors, have the highest incidence among primary brain tumors1. Solitary brain metastases (SBMs) are represented approximately 50% of all brain metastases and sometimes with unknown primary2. In real clinic practice, HGGs and SBMs share similar imaging features such as contrast enhancement pattern and extensive edema. Since the medical staging, clinic management and prognosis are indeed categorically distinct, it is clinically significant to distinguish HGGs from SBMs and benefit to more effective healthcare3. Although the postoperative histopathological examination is considered the gold standard for diagnosing SBMs from HGGs, its application is limited due to its invasive procedure. As is well known, malignant brain tumors usually present with peritumoral vasogenic edema and appear hyperintense signals on T2-weighted/fluid attenuation inversion recovery (T2W/FLAIR) MR images in the white matter surrounding the enhancing tumor--often called the peritumoral brain zone (PBZ). Studies have confirmed that HGGs tumoral foci principally infiltrates along white matter fiber tracts pathways within the peritumoral edema area, thus beyond the visibly contrast-enhancing border of the tumor4, 5. Conversely, the peritumoral of the SBMs was just presented as pure vasogenic edema and absence of infiltrative tumoral structure. In recent years, a multi-contrast and one-stop relaxation quantitative technique called synthetic MRI (SyMRI) using the magnetic resonance image compilation (MAGiC) has emerged, which can simultaneously generate multi-quantitative relaxation maps [synthetic relaxometry (T1 and T2), proton density (PD), etc.]6, 7. The aims of this study were to investigate whether relaxation maps generated from SyMRI is useful for differentiating HGGs from SBMs in PBZ.Material and Methods
A total 53 patients ranging from 35 to 76 years old (55.62 ± 10.27 years) were enrolled between August 2020 and September 2021. All lesions were confirmed by biopsy or surgical pathology. All MR examinations were performed with a 3.0T MR scanner (SIGNATM Architect, GE Healthcare, USA) equipped with a 48-channel head-neck unite coil. An axial MAGIC sequence for the SyMRI was scanned and acquired with the following parameters: TE = 4214msec, TR = 21.6msec, field of view (FOV) = 240 × 240mm2, matrix = 320 × 256, bandwidth = 22.76kHz, echo-train length = 16, slice thickness/gap = 5.0/1mm, NEX = 1, number of slices = 20, scan time = 3:36minutes. Post-contrast MAGiC sequence (MAGiC+C) acquisition was initiated 90s after contrast agent injection. All processing of the SyMRI data was directly performed using MR scanner host post-software (MAGiC v.100.1.1). The PBZ was considered as peritumoral T2-hyperintense regions within 1cm surrounding the enhancing components of the tumor. The T1, T2, and PD values in the ROI were automatically calculated from relaxation maps before (T1native, T2native, and PDnative) and after (T1post) injection of the contrast agent. The ΔT1ratio [(T1native–T1post)/T1native] values were manually calculated. Comparisons between groups were performed accordingly, with either a Student t test or a Mann-Whitney U test. Receiver operating characteristic (ROC) curves (AUC) were also evaluated to assess the diagnostic value of parameters for discrimination.Results
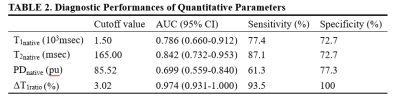
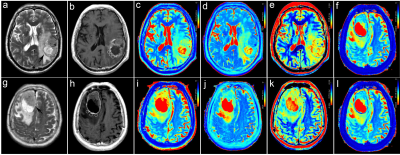
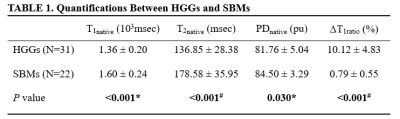
A total of 53 patients (31 with HGGs and 22 with SBMs) met the eligibility criteria. Representative images are shown in Figure 1 and there were significant differences in T1native, T2native, PDnative, and ΔT1ratio values between the HGGs and SBMs group (all P < 0.05, Table 1). The T1native, T2native, and PDnative values of the HGGs were significantly lower than those of the SBMs (T1native, 1.36 ± 0.20 × 103msec vs. 1.60 ± 0.24 × 103msec; T2native, 136.85 ± 28.38 msec vs. 178.58 ± 35.95 msec; PDnative, 81.76 ± 5.04pu vs. 84.50 ± 3.29pu). HGGs had significantly higher ΔT1ratio values than SBMs (P < 0.001). The ROC analysis results are shown in Table 2. ΔT1ratio presented the largest AUC of 0.974 which can achieve 90.3% sensitivity and 100% specificity for identifying HGGs, followed by T2native (AUC = 0.842), T1native (AUC =0.786), and PDnative (AUC = 0.699). The T1native, T2native, and PDnative values showed a similar AUC in differentiating HGGs from SBMs (all P > 0.05), which were lower than the AUC of the ΔT1ratio values (all P ≤ 0.025).Discussion
T1, T2 relaxometry and PD may reflect the inherent properties of matter and hence have the potential to serve as novel noninvasive biomarkers for different pathological properties. Blystad8 presented that R1 (1/T1). and R2 (1/T2) in the peritumoral edema decreased with the increase of the distance from the enhanced part of tumor and the gradient change of R1 was more obvious after enhancement, which may reflect the tumor infiltration. This is also confirmed in current study. Moreover, we found significant differences in T1native, T2native, PDnative, and ΔT1ratio regarding peritumoral edema in HGGs versus SBMs. One of the major limitations is the sample size for this study is relatively small. Therefore, larger studies are needed to confirm these results.Conclusion
Quantitative relaxation maps from synthetic MRI have potential for differential diagnosis of HGGs and SBMs with pathological confirmation.Acknowledgements
No acknowledgement found.References
1. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a ‘‘state of the science’’ review. Neuro Oncol 2014;16(7):896–913.
2. Bauer AH, Erly W, Moser FG, et al. Differentiation of solitary brain metastasis from glioblastoma multiforme: a predictive multiparametric approach using combined MR diffusion and perfusion. Neuroradiology 2015;57(7):697–703.
3. Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 2017;19(2):162–74.
4. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol 2007;114(5):443–458.
5. Stein AM, Demuth T, Mobley D, et al. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys J. 2007;92(1):356–365.
6. Gao W, Zhang S, Guo J, et al. Investigation of Synthetic Relaxometry and Diffusion Measures in the Differentiation of Benign and Malignant Breast Lesions as Compared to BI‐RADS. J Magn Reson Imaging 2021;53(4):1118-27.
7. Zhao L, Liang M, Wang S, et al. Preoperative evaluation of extramural venous invasion in rectal cancer using radiomics analysis of relaxation maps from synthetic MRI. Abdom Radiol (NY). 2021 46(8):3815-3825.
8. Blystad I, Warntjes JBM, Smedby Ö, et al. Quantitative MRI for analysis of peritumoral edema in malignant gliomas. PLoS One. 2017 12(5):e0177135.
Figures
Data are mean ± standard deviation.
P values less than 0.05 were considered to indicate statistical significance.
*P value represents the comparison results of HGGs and SBMs using the t-test analyzation.
#P value represents the comparison results of HGGs and SBMs using the Mann-Whitney U-test.
T1 = T1 relaxometry; T2 = T2 relaxometry; PD = proton density; ΔT1ratio = (T1native–T1post)/T1native.