3621
Deep Learning for Prediction of Recurrence in Nonfunctioning Pituitary Macroadenomas: Combination of Clinical and MRI Features1Department of Medical Imaging, Chi Mei Medical Center, Tainan, Taiwan, 2Chia Nan University of Pharmacy and Science, Tainan, Taiwan, 3Department of Electrical Engineering, National Cheng Kung University, Tainan, Taiwan, 4Graduate Institute of Electronics Engineering, National Taiwan University, Taipei, Taiwan, 5Department of Radiological Sciences, University of California, Irvine, CA, United States, 6Department of Radiology, E-Da Hospital and I-Shou University, Kaohsiung, Taiwan
Synopsis
A subset of nonfunctioning pituitary macroadenomas (NFMAs) show early progression/recurrence (P/R) after surgery. In clinical practice, one of the main challenges in the treatment of NFMAs is to determine factors that associated with P/R. This study investigated the role of deep learning for the prediction of P/R in NFMAs. 78 patients diagnosed with NFMAs were included. The hybrid CNN-MLP model using both clinical and MRI features showed the best performance for prediction of P/R in NFMAs, with accuracy of 84%, precision of 88%, and AUC of 0.87.
Background and Purpose
Pituitary tumors constitute 10%-15% of all primary brain tumors [1]. The nonfunctioning pituitary macroadenomas (NFMAs) (tumor diameter larger than 10 mm) is the most common type of pituitary tumors [2]. Although most NFMAs are classified as benign adenoma based on the 2017 WHO classification system [3] , a subset of NFMAs may showed early progression/recurrence (P/R) after surgical resection [4]. In clinical practice, gross-total resection (GTR) by trans-sphenoidal approach (TSA) is the optimal treatment for NFMAs; however, this aim is often difficult to achieve for large and solid NFMAs [5]. Conventional MR imaging findings such as invasion of the cavernous sinus, tumor size, and absence of tumor apoplexy had been reported as the important parameters related to P/R in NFMAs [6]. However, applications of deep learning (DL) for prediction of P/R in NFMAs has rarely been mentioned. The purpose of this study is to investigate the roles of DL for the prediction of P/R in NFMAs, with combination of clinical and MRI features in multilayer perceptron (MLP) and convolutional neural network (CNN) architectures.Materials and Methods
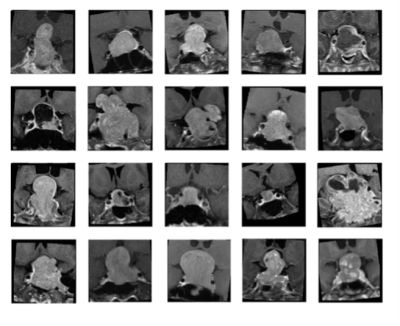
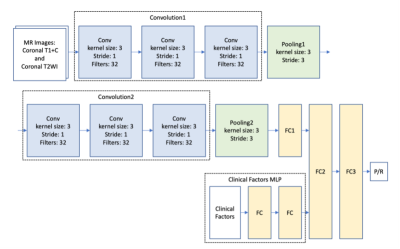
Seventy-eight patients (age 18 - 80 years; median age, 53.5 years) included in this study were diagnosed with NFMAs by MRI and pathological confirmation. The median follow-up time was 42 months (range 12 - 115 months). Total of 42 (42/78, 53.8%) patients were found to have P/R, and 36 patients remained stable disease. The MRI images were acquired using a 1.5T or a 3.0T scanner. Two MRI sequences include coronal T2WI and coronal contrast-enhanced (CE) T1WI were used for analysis. Python image-processing package (pydicom) [7] was applied to MRI dicom files to obtain pixel data. An experienced neuroradiologist selected one coronal CE T1WI slice showing the largest tumor height as the input image. We split the dataset into 5 folds for cross-validation. Data augmentations, including random flip, random rotate, random scale, and random shift are applied to each MR image to enhance the training effectiveness and prevent overfitting [8]. Some samples of processed images are shown in the Fig. 1. Because of small amount of medical data, we proposed a relatively light model based on two classical CNN architectures: LeNet [9] and VGG16 [10] (Fig. 2). For imaging analysis in CNN, coronal T2WI and CE T1WI were used. The two convolution layers in LeNet is replaced by convolution blocks from VGG16 (i.e., Convolution 1 and Convolution 2), which are formed by three 3 x 3 convolution layers (Fig. 2). The extracted image feature from second pooling layer (Pooling 2) is fed to three fully connected (FC) layers (FC1, FC2, and FC3) to predict the P/R. To provide clinical variables to the model, a MLP network which takes clinical factors as input was added before second fully connected layer (FC2). The hybrid CNN-MLP model can capture both image and numerical clinical features. The following clinical variables were included in the MLP model: sex, age, clinical symptoms, hypopituitarism, hyperprolactinemia, EOR, chiasmatic decompression, Knosp and Hardy classifications, compression of optic chiasm and 3rd ventricle, hydrocephalus, tumor diameter, and tumor volume.Results
P/R was diagnosed in forty-two (42/78, 53.8%) patients. Significant difference (p < 0.05) was observed in visual disturbance, hypopituitarism, EOR, successful chiasmatic decompression, cavernous sinus/extrasellar extension, compression of the optic chiasm/3rd ventricle, and tumor height/volume between patients with and without P/R (Fig. 3). Total 62 training cases and 16 validation cases from real patients were included. The data were extended to 6,240 training samples and 1,560 validation samples for machine learning. The evaluation metrics include accuracy, precision, recall, F1 score, loss, and AUC in training and validation sets. Among different combinations of inputs and model architectures, the hybrid light-weighted CNN_v1 model (using CE T1WI and T2WI) combined with 2-layer MLP (using clinical features) showed the best performance for prediction of P/R. In this predictive model, accuracy of 84%, precision of 88%, recall of 82%, F1 score of 0.85, and AUC of 0.87 were obtained in validation set.Conclusions
This study explored the effectiveness of DL in predicting P/R of the NFMAs. Even with a limited data set, the prediction model could achieve an accuracy of 84% and AUC of 0.87 for predicting recurrence in NFMAs. Better predictive performance was observed in a hybrid CNN-MLP model incorporating both clinical and MRI data as compared with classifiers using either clinical or MRI data alone. The results offer helpful information for the treatments planning in NFMAs.Acknowledgements
NoneReferences
1. Ostrom QT, et al. (2013) CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 15 Suppl 2:ii1-56.
2. Molitch ME. (2008) Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinology and metabolism clinics of North America 37 (1):151-171.
3. Lloyd RV, et al. (2017) WHO Classification of Tumours of Endocrine Organs ed 4. IARC Press, Lyon.
4. Roelfsema F, et al. (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15 (1):71-83.
5. Boxerman JL, at al. (2010) Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR American journal of roentgenology 195 (3):720-728.
6. Brochier S, et al. (2010) Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. European journal of endocrinology 163 (2):193-200.
7. Mason D, et al. (2011) SU-E-T-33: pydicom: an open source DICOM library. Medical Physics, 38:3493.
8. Paul J, et al. (2017) Deep learning for brain tumor classification. SPIE Medical Imaging. Vol. 10137.
9. Lecun Y, et al. (1998) Gradient-based learning applied to document recognition. Proceedings of the IEEE 86 (11):2278-2324.
10. Simonyan K, et al. (2015) Very deep convolutional networks for large-scale image recognition. Computational and Biological Learning Society. p. 1-14.
Figures