3618
Are topographically segregated excitatory neurons in visual thalamus functionally diverse? An optogenetic fMRI study1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
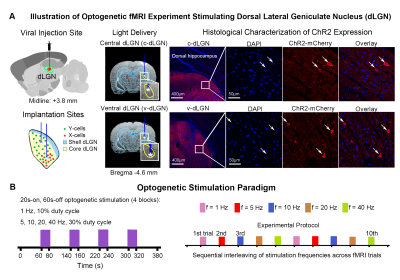
The dorsal lateral geniculate nucleus (dLGN) plays an essential role in visual processing. There are two types of topographically segregated excitatory neurons in dLGN with different outputs to visual cortex, suggesting functional differences when processing visual inputs at the subcortical thalamic level. However, their long-range functional pathways have yet to be reported. Here, we employed optogenetics in combination with fMRI to precisely target the two subdivisions of dLGN and examine whether these two types of neurons are truly functionally diverse at the systems level to facilitate various known complex visual processing functions.
Introduction
Vision is the sense humans rely on most to navigate the world and perform complex tasks1. Understanding how humans see represents one of the most fundamental goals of neuroscience. Rodent models are presently the preeminent model for studying the visual system2. The dorsal lateral geniculate nucleus (dLGN) is traditionally regarded as the principal conduit for visual information from the retina to visual cortex3. Although dLGN is known traditionally as a relay station, studies have shown that dLGN plays a vital role in complex multimodal information processing4 and spatial cognition5. The complexity of dLGN functions in vision is clearly visible in its neuronal cytoarchitecture3,4,6,7. The core of dLGN consists of two distinct morphological classes of excitatory relay neurons that are topographically segregated, namely X- and Y-cells8-10. Specifically, Y-cells are dispersed throughout the whole central core of dLGN (c-dLGN), while X-cells are almost exclusively located in the ventral region of core dLGN (v-dLGN)9. Both cells are known to project differently to the visual cortex6,8,11, suggesting functional diversity in visual processing at the thalamic level, not just the cortex. However, these works only focused on the geniculo-cortical pathway to visual cortex. Consequently, whether, where and how neural activity from topographically segregated dLGN neurons propagates beyond the primary visual cortex to subserve similar/different functions remains unresolved.In this study, we separately and precisely stimulated the two subdivisions of core dLGN (i.e., c-dLGN and v-dLGN) using optogenetics. We then utilized functional MRI (fMRI) to examine their respective functional pathways and spatiotemporal characteristics at the whole-brain level.
Method
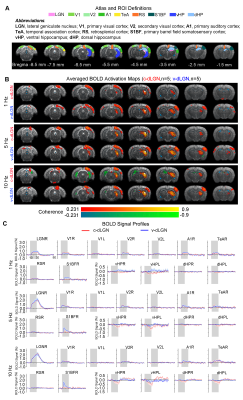
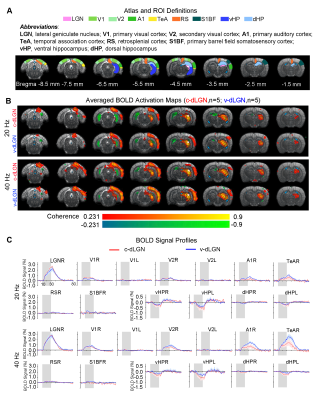
Animal preparation and optogenetic stimulation: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to the center of dLGN (-4.6 mm posterior to Bregma, +3.8 mm medial-lateral right hemisphere, -5.2mm from surface of dura) of adult SD rats (200-250g, male, 6-7 weeks old, n=10). Four weeks after injection, five rats were implanted with an opaque optical fiber cannula (d=250μm) at the c-dLGN, while the remaining five were implanted at v-dLGN (Figure 1A). Blue (473nm) light was presented to animals expressing ChR2 at 1Hz (10% duty cycle, 40mW/mm2), 5, 10, 20 and 40Hz (30% duty cycle, 40mW/mm2) in a block-design paradigm (Figure 1B). Stimulation frequencies were sequentially interleaved across fMRI trials.fMRI acquisition and analysis: fMRI data was acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 16 contiguous slices with 1mm thickness). Standard fMRI preprocessing was performed before the coherence analysis12 was applied to identify significant BOLD responses (p<0.001). BOLD signal profiles were extracted from atlas-defined ROIs.
Results
Optogenetic stimulation of both ipsilateral c-dLGN and v-dLGN groups at all frequencies evoked ipsilateral positive BOLD responses in stimulated region (i.e., LGN) and numerous sensorimotor cortices and higher order cortices associated with cognition, including primary and secondary visual (V1 & V2), primary auditory (A1), temporal association (TeA) and retrosplenial (RS) cortices (Figures 2 and 3). No obvious BOLD response differences were found in ipsilateral V1, V2, A1, TeA, and RS regions at low frequency stimulations below 10Hz in both groups (Figure 2B, C). Further, bilateral V1 and V2 responses were detected at 1Hz in both animal groups, albeit the contralateral responses were weak.Optogenetic excitation of v-dLGN evoked BOLD activations at somatosensory cortex, while c-dLGN evoked ventral hippocampal activations: Notably, the ipsilateral primary barrel field somatosensory (S1BF) cortex was activated only in the v-dLGN group. 1Hz stimulation evoked weaker BOLD responses within S1BF than 5 and 10Hz stimulation in v-dLGN group (Figure 2B, C). Ipsilateral S1BF responses in v-dLGN were no longer apparent at high frequency stimulation (Figure 3B, C). Interestingly, we observed strong negative BOLD responses in bilateral ventral hippocampus (vHP) and contralateral dorsal hippocampus (dHP) in c-dLGN group under high-frequency stimulation (10-40Hz), while negative BOLD responses were only found at bilateral vHP in v-dLGN group, which were significantly weaker than c-dLGN stimulation.
Optogenetic stimulation of c-dLGN and v-dLGN evoked different auditory cortex BOLD activations and responses: We further found that the BOLD responses in A1 and TeA in v-dLGN group were approximately twice as high as that in c-dLGN group during 40Hz stimulation (Figure 3B, C).
Discussion and Conclusion
We revealed that optogenetically stimulating the two distinct subdivisions of dLGN evoked brain-wide functional pathways within and beyond the primary output target (i.e., V1 and V2). Numerous studies have shown that cross-modal sensory communication is facilitated by the direct and reciprocal connections between primary sensory cortices, including V1, A1, and S1BF13-16. Our findings indicate that X-cells in the v-dLGN, not Y-cells in c-dLGN, are likely dominant in the visual-somatosensory interactions, particularly at low to mid frequencies (1-10Hz). Meanwhile, the more robust bilateral negative BOLD responses in vHP evoked at high-frequency stimulation (10-40Hz) of c-dLGN indicate a direct functional pathway with hippocampus at the thalamic level, especially from c-dLGN. Further, the more robust A1 activation induced by X-cells during high-frequency stimulation (40Hz) suggests that v-dLGN can significantly influence communication between V1 and A1 during multisensory processing.In conclusion, our optogenetic fMRI study reveals for the first time the differences in long-range functional pathways between two topographically segregated dLGN neurons at the systems level. The findings from this study will expand upon our current understanding of the complex role played by dLGN beyond simple relay of visual information.
Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L., and R7003-19F, HKU17112120 and HKU17127121 to E.X.W.), Lam Woo Foundation, Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
1. Milner, D. & Goodale, M. The visual brain in action, (OUP Oxford, 2006).
2. Seabrook, T.A., Burbridge, T.J., Crair, M.C. & Huberman, A.D. Architecture, Function, and Assembly of the Mouse Visual System. Annu Rev Neurosci 40, 499-538 (2017).
3. McAlonan, K., Cavanaugh, J. & Wurtz, R.H. Guarding the gateway to cortex with attention in visual thalamus. Nature 456, 391-394 (2008).
4. Okigawa, S., et al. Cell type- and layer-specific convergence in core and shell neurons of the dorsal lateral geniculate nucleus. Journal of Comparative Neurology 529, 2099-2124 (2021).
5. Diamanti, E.M., et al. Spatial modulation of visual responses arises in cortex with active navigation. Elife 10, e63705 (2021).
6. Guido, W. Development, form, and function of the mouse visual thalamus. J Neurophysiol 120, 211-225 (2018).
7. Monavarfeshani, A., Sabbagh, U. & Fox, M.A. Not a one-trick pony: Diverse connectivity and functions of the rodent lateral geniculate complex. Vis Neurosci 34, E012 (2017).
8. Halassa, M.M. & Sherman, S.M. Thalamocortical Circuit Motifs: A General Framework. Neuron 103, 762-770 (2019).
9. Krahe, T.E., El-Danaf, R.N., Dilger, E.K., Henderson, S.C. & Guido, W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. Journal of Neuroscience 31, 17437-17448 (2011).
10. Sherman, S.M. & Guillery, R.W. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357, 1695-1708 (2002).
11. Humphrey, A., Sur, M., Uhlrich, D. & Sherman, S. Projection patterns of individual X‐and Y‐cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. Journal of Comparative Neurology 233, 159-189 (1985).
12. Lee, J.H., et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
13. Klinge, C., Eippert, F., Röder, B. & Büchel, C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. Journal of Neuroscience 30, 12798-12805 (2010).
14. Lohse, M., Dahmen, J.C., Bajo, V.M. & King, A.J. Subcortical circuits mediate communication between primary sensory cortical areas in mice. Nature Communications 12, 1-14 (2021).
15. Knöpfel, T., et al. Audio-visual experience strengthens multisensory assemblies in adult mouse visual cortex. Nature Communications 10, 1-15 (2019).
16. Powers,
A.R., Hevey, M.A. & Wallace, M.T. Neural correlates of multisensory
perceptual learning. Journal of
Neuroscience 32, 6263-6274
(2012).
Figures