3617
How does midazolam affect resting-state networks in common marmosets? An investigation using a 9.4 T magnetic resonance imaging system.1Tokyo Metropolitan University, Tokyo, Japan, 2The Jikei University School of Medicine, Tokyo, Japan, 3RIKEN Center for Brain Science, Saitama, Japan, 4Keio University, Tokyo, Japan, 5Kyoto University, Aichi, Japan, 6Central Institute for Experimental Animals, Kanagawa, Japan
Synopsis
The purpose of this study is to evaluate the effects of midazolam on resting-state networks in common marmosets. Functional data were collected using a 9.4 T MRI system in the sedative condition with midazolam, the awake condition as a positive control, and the anesthetic condition with isoflurane as a negative control. Independent component analysis and functional connectivity analysis were performed. These results were apparently altered by isoflurane from awake condition, but not largely by midazolam. These results in midazolam were different from a previous report in humans and it may cause different effects of midazolam in human and nonhuman primates.
INTRODUCTION
In recent years, the idea that consciousness is formed by brain networks has become increasingly pervasive. Unconsciousness with sedative agents is thus thought to occur due to the collapse of these brain networks following dissociation of intercellular communication1. Loss of consciousness by sedative agents is commonly observed in various species, but some gamma-aminobutyric acid (GABA) receptor agonists only sedate humans, having no effects in nonhuman primates (NHP) even though they are closely related to humans2. This phenomenon suggests the existence of human-specific neural systems and activities that create consciousness. Investigation of this species difference may reveal highly developed human brain functions and explain how human brains differ from NHP brains. In this study, the effects of midazolam, a GABA receptor agonist, on resting-state networks (RSNs) in NHPs were evaluated using common marmosets (Callithrix jacchus) and a 9.4 T magnetic resonance imaging (MRI) system.METHODS
This study was approved by the Animal Experiment Committees of the RIKEN Center for Brain Science and conducted in accordance with institutional Guidelines for Conducting Animal Experiments. Three male and three female healthy marmosets aged 3–6 years were included in this study. Functional data were measured in the sedative condition with midazolam (Mid condition), in the awake condition as a positive control (Awake condition), and in the anesthetic condition with isoflurane as a negative control (Iso condition). In the Mid condition, midazolam was administered through an indwelling needle inserted in a tail vein. A dose of 0.25 mg/kg of midazolam was initially administered and continuous infusion at a rate of 0.6 mg/kg/h was performed during data collection. In the Iso condition, isoflurane was initially administered with a facial mask and the marmosets were intubated with an intratracheal tube after sufficient sedation was obtained. General anesthesia was maintained with 1.8% isoflurane during data collection. All data were collected with a 9.4 T MRI scanner (BioSpec 94/30; Bruker BioSpin, Ettlingen, Germany). Imaging was performed with the following parameters: gradient spin echo (EPI); TR/TE = 2,000/16 ms; repetition time = 150; isotropic resolution = 700 µm; acquisition time = 60 min. The functional magnetic resonance images (fMRI) were preprocessed (top-up with FSL and realign, normalize, and smoothing with SPM12). Independent component analysis (ICA) was performed to detect RSNs. Partial correlation coefficients (PCCs) and betweenness centrality were calculated among brain regions of gray matter which divided into 52 regions per hemisphere (Figure 1) to evaluate the effects of midazolam on each brain region.RESULTS
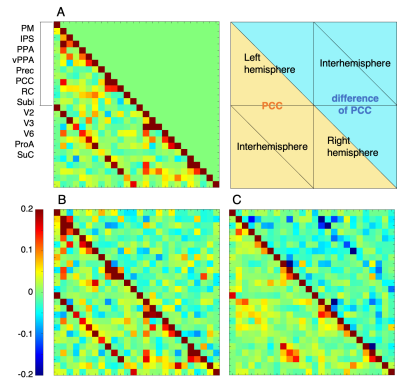
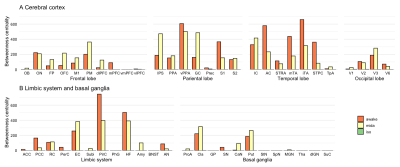
In the Mid condition (Figure 2), 17 RSNs were detected; in contrast, 19 RSNs and 7 RSNs were detected in the Awake and Iso conditions, respectively. In the Mid condition, a decrease in relating voxels in the frontal pole network and disappearance of the caudal visual network and thalamic and temporal lobe network were observed. In contrast to these network decreases, the auditory network signal was increased. Functional connectivity (FC) matrices were formed from PCCs. The FC matrices of regions detected as the default mode network (DMN) by ICA are shown in Figure 3. PCCs tended to decrease in the Mid condition; however, no large alternation was observed between the Awake and Mid conditions and FCs were well preserved—especially within a lobe. On the other hand, FC decreased in the Iso condition, which is a negative control, especially within a lobe and between hemispheres. The betweenness centrality of each brain region in each condition is shown in Figure 4. In the Mid condition, betweenness centrality tended to increase in the frontal lobe and decrease in the temporal lobe, but the function as a hub was generally preserved. In contrast, almost no betweenness centrality was detected in the Iso condition.DISCUSSION
The results of this study suggest that the effects of midazolam on RSNs in marmosets are small. In a similar previous study of humans, it was reported that FC in RSNs related to lower-level sensory networks, such as DSN, AN, and PVN, was increased, but RSNs related to higher cognitive functions, such as DMN and DAN, were impaired by midazolam3. Our finding that the auditory network signal was increased in marmosets is consistent with this report, but the results that higher cognitive networks were well preserved do not agree with this report. These findings suggest that midazolam can depress function only in human cortical areas. Nørgaard et al. showed that the benzodiazepine binding site, where midazolam binds to GABA receptors, is well detected in cortical areas in humans4. This deviation in expression may result in differences in the effects of GABA receptor agonists between humans and NHPs.CONCLUSION
In this study, we showed that midazolam has fewer effects on the marmoset brain compared with the human brain—especially in terms of higher cognitive functions. These results suggest there are species differences and that human-specific neural activity exists in the cortical region to create higher-order brain functions. Further studies about receptor expression and affinity in marmosets are needed to support these findings.Acknowledgements
References
1. Alkire, M. T., Hudetz, A. G. & Tononi, G. Consciousness and anesthesia. Science (New York, N.Y.) 322, 876–80 (2008).2. Miyabe, T., Nishimura, R., Mochizuki, M., Sasaki, N. & Mastubayashi, K. Chemical restraint by medetomidine and medetomidine-midazolam and its reversal by atipamezole in Japanese macaques (Macaca fuscata). Veterinary Anaesthesia and Analgesia 28, 168–174 (2001).
3. Liang, P. et al. Disruption of cortical integration during midazolam‐induced light sedation. Human Brain Mapping 36, 4247–4261 (2015).
4. Nørgaard, M. et al. A high-resolution in vivo atlas of the human brain’s benzodiazepine binding site of GABAA receptors. Neuroimage 232, 117878 (2021).
Figures
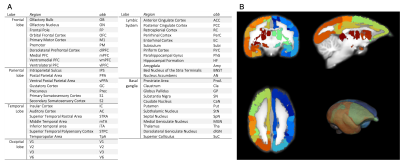
52 regions of the cerebral gray matter and its abbreviations (A) and actual divided brain image (B). The hemisphere was divided into 52 regions for analysis.
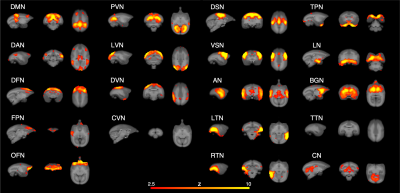
RSNs detected in Mid condition. DMN; default mode network, DAN; dorsal attention network, DFN; dorsal frontal network, FPN; frontal pole network, OFN; orbitofrontal network, PVN; primary visual network, LVN, lateral visual network, DVN; dorsal visual network, CVN; caudal visual network, DSN; dorsal somatomotor network, VSN; ventral somatomotor network, AN; auditory network, LTN; left temporal network, RTN; right temporal network, TPN; temporal pole network, LN; limbic network, BGN; basal ganglia network, TTN; thalamic and temporal lobe network, CN; cerebellar network.