3611
Disparate Multivariate Relationships between Brain-Behavior Dimensions across Patients with Schizophrenia at Different Illness Stages1Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Provinc, West China Hospital of Sichuan University, Chengdu, China
Synopsis
Clarifying brain-behavior associations in schizophrenia helps understand neurobiological mechanisms and explore biomarkers for patient stratification and cognition-targeted interventions. Sparse canonical correlation analysis (sCCA) is a method that maximizes the correlations between linear combinations of each high-dimensional data set, providing more information relative to traditional bivariate correlation analysis. In order to characterize multivariate brain-behavior associations in schizophrenia, we performed sCCA in patients at different illness stages. Disparate canonical correlations were found across FES patients and stable treated patients, involving cortical thickness and surface area contributed in each sample, consistent with their well-established differences in neurodevelopmental trajectories, genetics, and contributions to cognition.
Introduction
Neuroanatomic abnormalities and cognitive impairment are significant characteristics for patients with schizophrenia [1-3]. Clarifying brain-behavior associations helps in understanding neurobiological mechanisms in this disorder and discovering biomarkers for patient stratification and cognition-targeted interventions. In previous studies, bivariate brain-behavior correlations have been broadly investigated in schizophrenia [4-6]. A considerable limitation for this approach is that features within neuroanatomic or cognitive dimensions are treated as independent terms, ignoring their multiple complex interactions. In the present work, we performed sparse canonical correlation analyses (sCCA) between brain structures and cognitive function in two samples of patients with schizophrenia at different illness stages to explore their multivariate brain-behavior associations.Materials and Methods
Subjects: In order to characterize multivariate associations between brain-behavior dimensions, we included two samples of patients with schizophrenia at different illness stages, including 59 drug-naïve patients with first-episode schizophrenia (FES) (age: 28.46 ± 9.24 years; sex: 31 females; illness duration: 20.07 ± 37.44 months), and 115 stable antipsychotic-treated patients (age: 45.95 ± 7.10 years; sex: 41 females; illness duration: 229.30 ± 106.53 months; the daily dose of antipsychotics: 559.2 ± 312.33 mg/day). The Structured Clinical Interview of DSM-IV was applied for the diagnosis of schizophrenia.Cognitive function assessment: We used the Brief Assessment of Cognition in Schizophrenia (BACS) [7] to assess the cognitive performance of these patients from six dimensions such as verbal memory, working memory, motor speed, verbal fluency, attention and speed of information processing, and executive functioning.
Imaging acquisition and preprocessing: T1-weighted brain images were acquired from patients using two 3-T MR scanners in West China Hospital. For patients from the same cohort, they were scanned by the same scanner with identical scanning parameters. The FreeSurfer software (https://surfer.nmr.mgh.harvard.edu) version 6.0 was employed for cortical reconstruction and subcortical segmentation [8,9]. We extracted thickness and surface area from neocortical regions based on the Desikan-Killiany (DK) atlas [10]. Volumes from subcortical structures such as bilateral thalamus, putamen, caudate, pallidum, hippocampus, amygdala, and nucleus accumbens were also obtained.
Statistical analysis: As an algorithm used to explore multivariate associations between two high-dimensional data sets, sCCA [11,12] maximizes the correlation between linear combinations of each side. We conducted sCCA between neuroanatomic and cognitive features in each patient sample using R software version 4.0.2. Regressing out corresponding covariates and standardizing the residuals into z-scores were performed before analysis. Particularly, variance related to age, sex, and ICV was removed for neuroanatomic features, and age, sex, and education level was treated as nuisance variables for cognitive function. We employed permutation tests in order to detect the significance of any pair of canonical variates. For each significant pair of canonical variates, cross-loadings were threshold at 0.20 and subsequently extracted for visualization. Cross-loadings are the correlation between each feature and the opposite canonical variate.
Results
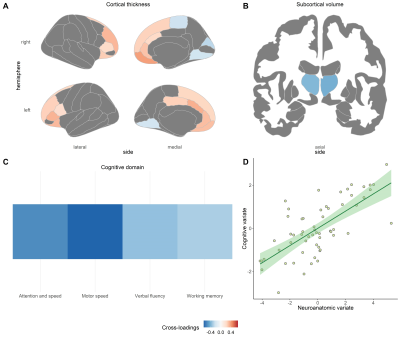
Disparate canonical correlations were found across FES and stable treated patients, involving cortical thickness and cortical surface area contributed in each sample, respectively.In drug-naïve FES patients, a significant pair of canonical variates were found between cognition and the combination of cortical thickness and subcortical volumes (sCCA r = 0.70, P = 0.004) (see Figure 1). The cognitive variate captured four dimensions of cognitive function, including attention and speed of information processing, motor speed, verbal fluency, and working memory (Figure 1C). Cortical thickness in the default, frontoparietal, and ventral attention networks was positively associated with the cognitive variate (Figure 1A), while thalamic volumes were negatively related to this cognitive variate (Figure 1B).
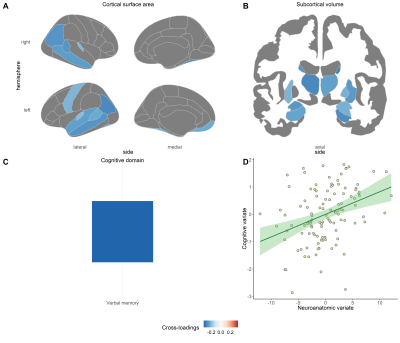
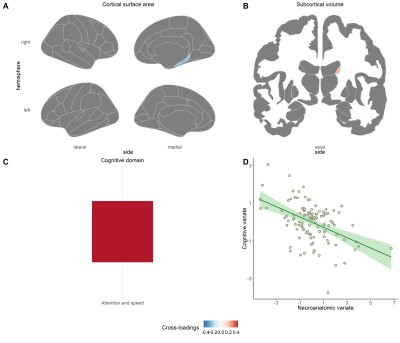
In stable treated patients, two significant pairs of canonical variates were found between cognition and the combination of cortical surface area and subcortical volumes (Pair 1: sCCA r = 0.36, P = 0.027; Pair 2: sCCA r = -0.56, P = 0.026) (see Figure 2 and 3). For the first one, the cognitive variate captured the domain of verbal memory (Figure 2C) and was negatively associated with cortical surface area in the default and the somatomotor network (Figure 2A) and volumes in the most subcortical regions (Figure 2B). Regarding the second pair of canonical variates, the cognitive variate was composed of attention and speed of information processing (Figure 3C) and was negatively related to surface area in the right parahippocampus gyrus (Figure 3A) and positively associated with volume in the right caudate (Figure 3B).
Conclusions
Disparate multivariate correlations were found in patients with schizophrenia at different illness stages, where cortical thickness measures contributed to associations in FES patients, and cortical surface area measures were prominent for correlations in stable treated patients. These findings are consistent with well-established differences between cortical thickness and cortical surface area in neurodevelopmental trajectories, genetics, and contributions to cognition. Our work provides new sight for brain-behavior associations in patients at different illness stages. It helps in understanding neurobiological mechanisms and discovering biomarkers for patient stratification and cognition-targeted interventions.Acknowledgements
No acknowledgement found.References
1 Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A Systematic and Meta-analytic Review of Neural Correlates of Functional Outcome in Schizophrenia. Schizophr Bull. 2017;43(6):1329-47.
2 Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107-12.
3 Gong Q, Lui S, Sweeney JA. A Selective Review of Cerebral Abnormalities in Patients With First-Episode Schizophrenia Before and After Treatment. Am J Psychiatry. 2016;173(3):232-43.
4 Li X, Black M, Xia S, Zhan C, Bertisch HC, Branch CA, et al. Subcortical structure alterations impact language processing in individuals with schizophrenia and those at high genetic risk. Schizophr Res. 2015;169(1-3):76-82.
5 Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep. 2018;8(1):1183.
6 Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Subcortical association with memory performance in schizophrenia: a structural magnetic resonance imaging study. Transl Psychiatry. 2018;8(1):20.
7 Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-97.
8 Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-55.
9 Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22.
10 Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-80.
11 Modabbernia A, Reichenberg A, Ing A, Moser DA, Doucet GE, Artiges E, et al. Linked patterns of biological and environmental covariation with brain structure in adolescence: a population-based longitudinal study. Mol Psychiatry. 2020.
12 Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9(1):3003.
Figures