3609
Disrupted topological organization in cerebral small vessel disease: A resting-state functional brain networks and graph theory study
Haotian Xin1, Hongwei Wen2,3, Mengmeng Feng1, Lingfei Guo4, and Changhu Liang4
1Cheeloo College of Medicine, Shandong University, Jinan, China, 2Key Laboratory of Cognition and Personality (Ministry of Education), Chongqing, China, 3School of Psychology, Southwest University, Chongqing, China, 4Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
1Cheeloo College of Medicine, Shandong University, Jinan, China, 2Key Laboratory of Cognition and Personality (Ministry of Education), Chongqing, China, 3School of Psychology, Southwest University, Chongqing, China, 4Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Synopsis
We aim to investigate functional topological brain networks changes in cerebral small vessel disease (CSVD) patients with and without cerebral microbleeds (CMBs). We divided the subjects into three subgroups: CSVD with CMBs (CSVD-c), CSVD without CMBs (CSVD-n) and control. Then, we analyzed topological disruptions between groups using graph theory and related these alterations to clinical parameters. CSVD-c showed significantly altered global, nodal topological properties and disrupted distribution of hub regions, involving the default mode network (DMN), attention, sensorimotor and visual functional areas. These findings extended our understanding of neurobiological mechanisms of CSVD.
Abstract
IntroductionCerebral small vessel disease (CSVD) seriously endangers public health and the ratio of dementia caused by CSVD can be as high as 36% to 67% in this decade 1, 2. As one of imaging markers of CSVD, cerebral microbleeds (CMBs) could be detected in T2-weighted gradient-recalled echo (GRE) or susceptibility-weighted imaging (SWI) sequences 3. CMBs have a significant impact on the cognitive function of CSVD patients and is an independent risk factor 4, 5. However, the neurobiological mechanism of CMBs leading to cognitive impairment (CI) in CVSD patients remains unclear. Accumulated resting-state functional MRI (rs-fMRI) studies have mainly divided subjects into CSVD with and without CI or focused on white matter lesions 6, 7. Additionally, very few studies used graph theory to explore the topological organization of the whole-brain connectome in CSVD patients. Given that, we aim to investigate the topological alterations in functional brain networks and to assess the relationship between functional impairment and topological network changes in CSVD patients with and without CMBs, which could advance our current understanding of the pathophysiological mechanisms underlying CSVD.
Methods
24 CSVD with CMBs (CSVD-c) patients, 42 CSVD without CMBs (CSVD-n) patients and 36 healthy control (HC) subjects were recruited from outpatient clinics in Shandong provincial hospital affiliated to Shandong first medical university from September 2018 to June 2019. All subjects were tested on their levels of cognitive function, covering major areas of cognitive function. All subjects underwent 3-T MRI, including three-dimensional T1-weighted (T1W), blood oxygen level-dependent (BOLD) and SWI. In addition, T2-weighted (T2W) turbo spin echo, T2W fluid attenuated inversion recovery (FLAIR), diffusion-weighted were acquired to detect brain abnormalities. Rs-fMRI data reprocessing was performed using statistical parametric mapping and region-based functional connectivity network was constructed. Both network topological properties including five small-world property metrics, two network efficiency metrics and a regional measure were calculated using a graph theoretical network analysis toolbox. Then we calculated the area under the curve (AUC) for each topological property over the range of sparsity. Once significant inter-group differences were identified in any regional topological metrics, we further assessed the correlations between these metrics and clinical parameters in the CSVD-c and CSVD-n groups, respectively.
Results
The demographic and clinical characteristics of each group are summarized (Figure 2). All groups exhibited the high-efficiency small-world topology characterized and alterations in the global properties of functional networks of each group are summarized (Figure 3). We found partially similar hub distributions among groups, meanwhile, CSVD-c and CSVD-n patients showed different redistributions of the network's hubs (Figure 4). Significantly decreased and increased nodal betweenness centrality (BC) in multiple functional areas and correlations between network topological alterations to clinical parameters are summarized (Figure 5).
Discussion
The functional brain networks of three groups exhibited a small-world architecture, showing that the brain functional networks were constructed reliably. Previous studies showed the preservation of a small-world architecture in healthy population and in the presence of pathology 8, 9. Of note, compared with CSVD-n and the HCs, only CSVD-c patients showed significantly decreased global efficiency (Eglob), local efficiency (Eloc), clustering coefficient (Cp) and increased shortest path length (Lp), indicating disrupted topological organizations of brain networks in CSVD-c patients, which can be attributed to affected functional connections 10, 11. From the perspective of information transfer, these reflect a disbalance between local specialization and global integration 12. From the aspect of histopathologic correlates, CMBs are more likely to be induced by systemic inflammation, and higher level of inflammatory markers were found compared with CSVD-n patients, implying more severe pathological processes 13, 14.
Similar global hub distributions among groups were found, suggesting these key regions are conserved and the small-world networks can tolerate developmental alterations or disease 15. Meanwhile, partial reorganization appeared in CSVD patients, which can be interpreted as a balance effect. Specific metastases mainly occurred in attention and visual related structures 16, 17. Fusiform gyrus (FFG) and inferior frontal gyrus (IFG) are specifically in charge of visuoperceptual working memory 18. Right FFG lacked and additional right IFG appeared as hub regions in CSVD-n group compared with HC group. Thus, we speculate that CSVD impair the function of right FFG, which might be compensated by right IFG. CSVD patients exhibited significantly altered BC, mainly distributed in the default mode network (DMN), attention, visual, and sensorimotor systems. Especially, DMN plays an essential role in higher cognition, and accumulated literatures reported the related alterations of DMN in CSVD patients 6, 10, 19-21. Furthermore, the nodal BC of right posterior cingulate gyrus (PCG) serving as a core hub for DMN showed a significantly positive correlation with the symbol digit modalities test (SDMT) scores and negative correlation with the trail-making test (TMT) scores in CSVD patients 22. Thereby the decrease in BC of PCG indicates the decrease in attention and information processing speed evaluated by two tests above in CSVD patients.
Conclusion
Our study suggested that CSVD patients with and without CMBs had segregated disruptions in the topological organization of the intrinsic functional brain network, which may be due to different etiological contributions. These findings may extend our understanding of how functional disruptions of neuronal circuits link to the pathophysiology of CSVD.
Acknowledgements
We thank all of the volunteers and patients for their participation in our study.References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689-701.2. Grau-Olivares M, Arboix A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: a forerunner of vascular dementia? Expert Rev Neurother. 2009;9(8):1201-17.
3. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-38.
4. van Es AC, van der Grond J, de Craen AJ, et al. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77(15):1446-52.
5. Nannoni S, Ohlmeier L, Brown RB, et al. Cognitive impact of cerebral microbleeds in patients with symptomatic small vessel disease. Int J Stroke. 2021:17474930211012837.
6. Liu R, Wu W, Ye Q, et al. Distinctive and Pervasive Alterations of Functional Brain Networks in Cerebral Small Vessel Disease with and without Cognitive Impairment. Dement Geriatr Cogn Disord. 2019;47(1-2):55-67.
7. Schaefer A, Quinque EM, Kipping JA, et al. Early small vessel disease affects frontoparietal and cerebellar hubs in close correlation with clinical symptoms--a resting-state fMRI study. J Cereb Blood Flow Metab. 2014;34(7):1091-5.
8. Yi LY, Liang X, Liu DM, et al. Disrupted topological organization of resting-state functional brain network in subcortical vascular mild cognitive impairment. CNS Neurosci Ther. 2015;21(10):846-54.
9. Wang J, Chen Y, Liang H, et al. The Role of Disturbed Small-World Networks in Patients with White Matter Lesions and Cognitive Impairment Revealed by Resting State Function Magnetic Resonance Images (rs-fMRI). Med Sci Monit. 2019;25:341-56.
10. Yi L, Wang J, Jia L, et al. Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One. 2012;7(9):e44758.
11. Zhou X, Hu X, Zhang C, et al. Aberrant Functional Connectivity and Structural Atrophy in Subcortical Vascular Cognitive Impairment: Relationship with Cognitive Impairments. Front Aging Neurosci. 2016;8:14.
12. Liao X, Vasilakos AV, He Y. Small-world human brain networks: Perspectives and challenges. Neurosci Biobehav Rev. 2017;77:286-300.
13. Sumbria RK, Grigoryan MM, Vasilevko V, et al. Aging exacerbates development of cerebral microbleeds in a mouse model. J Neuroinflammation. 2018;15(1):69.
14. Miwa K, Tanaka M, Okazaki S, et al. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke. 2011;42(11):3202-6.
15. Wen H, Liu Y, Rekik I, et al. Disrupted topological organization of structural networks revealed by probabilistic diffusion tractography in Tourette syndrome children. Hum Brain Mapp. 2017;38(8):3988-4008.
16. Liu R, Chen H, Qin R, et al. The Altered Reconfiguration Pattern of Brain Modular Architecture Regulates Cognitive Function in Cerebral Small Vessel Disease. Front Neurol. 2019;10:324.
17. Spagna A, Hajhajate D, Liu J, et al. Visual mental imagery engages the left fusiform gyrus, but not the early visual cortex: A meta-analysis of neuroimaging evidence. Neurosci Biobehav Rev. 2021;122:201-17.
18. Sala-Llonch R, Palacios EM, Junqué C, et al. Functional networks and structural connectivity of visuospatial and visuoperceptual working memory. Front Hum Neurosci. 2015;9:340.
19. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38.
20. Andrews-Hanna JR, Reidler JS, Huang C, et al. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104(1):322-35.
21. Chen H, Li Y, Liu Q, et al. Abnormal Interactions of the Salience Network, Central Executive Network, and Default-Mode Network in Patients With Different Cognitive Impairment Loads Caused by Leukoaraiosis. Front Neural Circuits. 2019;13:42.
22. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76.
Figures
The
90 cortical and subcortical regions of interest defined in our study are shown.
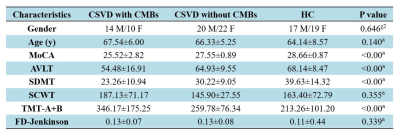
Demographic and clinical characteristics of CSVD
patients and controls are shown. Abbreviations: CSVD:
cerebral small vessel disease; CSVD-c: CSVD with CMBs; CSVD-n: CSVD without
CMBs; χ2: chi-square test; a: ANOVA test; MoCA: Montreal Cognitive Assessment;
AVLT: sum of Rey Auditory Verbal Learning Test (N1-7); SDMT: Symbol Digit
Modalities Test; SCWT: sum of Stroop Color-Word Test (stroop1-3); TMT: the
Trail-Making Test; TMT A+B: sum of TMT-A and TMT-B; FD_Jenkinson: frame-wise
displacement.
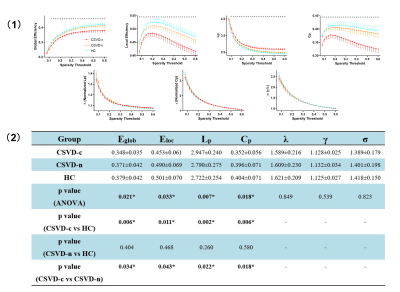
(1) Differences
in global topological properties of functional networks among three groups are
shown. Data
points marked with a star indicate a significant (p<0.05, ANOVA with LSD
post-hoc test) intergroup differences in the global network metric under a
corresponding sparsity threshold. (2) Group comparisons of AUC values of global
network properties are shown. Abbreviations: HC, healthy controls; *: p<0.05
(ANOVA, LSD post-hoc test).
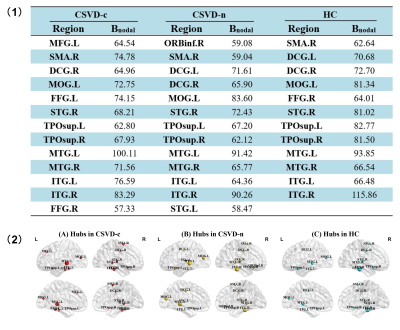
(1) Hub regions of functional
networks in both CSVD and control groups are shown. Abbreviations:
Bnodal represents the AUC value of nodal betweenness centrality
across thresholds. (2) Hub region distributions of the functional networks for both groups are
shown. The hub nodes are shown with different node sizes indicating
their nodal betweenness centrality values. The brain graphs were visualized by
using BrainNet Viewer software
(http://www.nitrc.org/projects/bnv/). For
the abbreviations of nodes, see Figure 1.
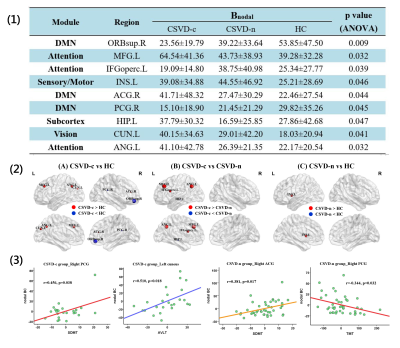
(1)
Brain regions showing significantly altered nodal BC among three groups are
shown. (2) The differences in nodal
BC of the functional networks among three groups are shown. The disrupted nodes with the
significantly decreased or increased nodal BC are shown in blue or red, and the
scaled node sizes indicate the F
values in ANOVA test. (3) Correlations
between nodal BC and clinical parameters are shown. The coordinate value of
both X-axis (clinical parameter) and Y-axis (nodal BC) consider age,
gender and mean FD as covariates. For the abbreviations
of nodes, see Figure 1.
DOI: https://doi.org/10.58530/2022/3609