3608
Altered neurovascular coupling in drug-naïve attention-deficit/hyperactivity disorder: Evidence from a comprehensive fMRI analysis1First Affiliated Hospital, Sun Yat-sen University, guangzhou, China, 2MR Research, GE Healthcare, guangzhou, China
Synopsis
As the most common childhood-onset neurodevelopmental disorder, the pathogenesis of attention-deficit/ hyperactivity disorder (ADHD) is still unclear. Non-invasive neuroimaging methods open new avenues to characterize developmental changes in the human brain. Neurovascular coupling (NVC) reflects the close relationship between neuronal activity and cerebral blood flow (CBF), which provides a new mechanistic insight into developmental brain alteration. We aim to investigate the potential NVC alteration in ADHD children.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neuropsychiatric disorder characterized by age-inappropriate inattention and hyperactivity/impulsivity [1]. The etiological bases and neural substrates of childhood ADHD are far from being fully understood. During development, the vasculature, neuronal circuits, and astrocyte networks integrate to form the neurovascular unit (NVU), and engage “NVC” mechanism. The brain regions with stronger connectivity tend to have higher intrinsic neuronal activities with more energy consumption, which results in increased cerebral blood flow [2]. Convergent neuroimaging studies [3-6] have discovered the reduced neural activity, changed cerebral perfusion in multiple grey matter (GM) regions, and related cognitive dysfunction in ADHD patients, based on the analyses of multiple aspects of blood oxygenation level dependent (BOLD) signals (ALFF, fALFF, ReHo, and DC) and the arterial spin labeling (ASL) technique (CBF). However, research rarely emphasizes the alterations of NVC in pediatric patients with drug-naïve ADHD by combining BOLD and ASL techniques. The knowledge about the underlying NVC pathophysiological mechanisms in ADHD are not fully understood. In current study, we utilized the alteration of both global-level and regional-level NVC to investigate the grey matter activity abnormalities in ADHD, to explore the specific cortex activity patterns of pathophysiological mechanisms in drug-naïve children with ADHD.Methods
Fitty drug-naïve pediatric ADHD and 42 age-, gender-, and handedness-matched healthy controls (HCs) were enrolled. The partial correlation coefficients between ASL and BOLD maps, defined as the NVC biomarkers (defined as CBF-ALFF, CBF-fALFF and CBF-DC coupling), which were measured at whole GM level and regional level based on automated anatomical labeling (AAL) atlas. Two-sample t-tests were used to compare the intergroup differences in NVC biomarkers. Relationships between the altered NVC biomarkers and clinical symptom severity were analyzed in the ADHD group.Results
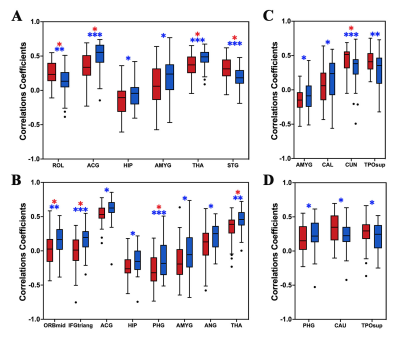
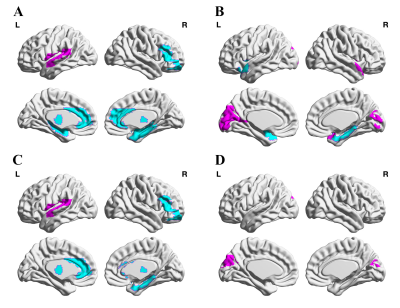
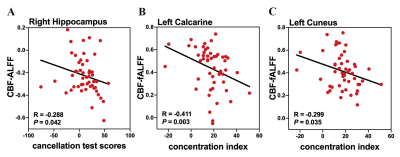
ADHD patients showed a significantly reduced global CBF-ALFF coupling (P < 0.001) compared to HCs at the voxel-level, but no significant differences of CBF-fALFF and CBF-DC coupling. With regard to regional NVC biomarkers, alterations of 19 brain regions with P value less than 0.05 (uncorrected) were found in ADHD patients. Furthermore, the regional CBF-ALFF coupling were significantly lower in bilateral thalamus and in the left anterior cingulate, right middle orbital frontal gyrus and right parahippocampal gyrus, as well as significantly increased CBF-ALFF coupling in the left rolandic operculum and left superior temporal gyrus (FDR-corrected). When regards to CBF-fALFF coupling, the increased profiles were found in left cuneus (FDR-corrected). Moreover, CBF-ALFF coupling of the right hippocampus, CBF-fALFF coupling of the left calcarine and left cuneus nodal efficiency were negatively correlated with clinical symptom severity of ADHD patients (all P <0.05).Discussion
There were three main findings in our article: (1) at the whole GM level, ADHD patients showed a significantly reduced averaged global CBF-ALFF coupling, while no significant between-group differences were found of CBF-fALFF and CBF-DC biomarkers; (2) at the GM regional level, neurovascular biomarkers in ADHD patients were significantly decreased in frontal, prefrontal or limbic-subcortical regions and were related to cognitive or reward circuits information processing; (3) CBF-ALFF in right hippocampus, CBF-fALFF in left calcarine and left cuneus was found to be negatively correlated with the symptom severity of individual ADHD patients.Previous genetic studies have been already suggested the involvement of the dopaminergic system dysfunction in patients with ADHD [7]. The alterations in dopaminergic systems affect the function of brain structures that moderate executive function, working memory, emotional regulation, and reward processing. We hypothesis that dopaminergic system dysfunction in related GM regions caused neurovascular biomarkers alterations. Apart from the global GM level NVC properties, specific regional NVC properties changes were also observed in ADHD compared with the HC group, which were mainly distributed in the regions of default-mode networks (DMN), salience network (SN), attention network (AN), visual network (VN) and bilateral thalamus (THA). These findings provide partial support for the “triple network model” of the pathophysiology associated with ADHD based on resting-state functional connectivity analysis that altered connectivity in ADHD is noted not only in the DMN, frontoparietal network (FPN) and AN, but also in reward-related and affective circuits [8].Conclusion
The neurovascular biomarkers in the ADHD group indicated a significantly reduced averaged global CBF-ALFF coupling, while abnormal neurovascular biomarkers in ADHD patients were significantly decreased in frontal, prefrontal or limbic-subcortical regions and were related to cognitive or reward circuits information processing. The novel findings reveal that altered neurovascular coupling may be a potential neural mechanism in ADHD patients.Acknowledgements
This work was supported by the Natural Science Fund Youth Science Fund Project of China [grant number 82001439], the Medical Scientific Research Foundation of Guangdong Province [grant number A2020327] and the Guangdong Basic and Applied Basic Research Foundation, China (No.2020A1515011436). We would like to thank the participants and their families and the staff at the MRI at the First Affiliated Hospital of Sun Yat-sen University for all their help and support.References
1. Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. The Lancet 387:1240-12502. Iadecola C (2017)
2. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96:17-423. Jiang K, Wang J, Zheng A et al (2020)
3. Amplitude of low-frequency fluctuation of resting-state fMRI in primary nocturnal enuresis and attention deficit hyperactivity disorder. Int J Dev Neurosci 80:235-2454. An L, Cao QJ, Sui MQ et al (2013)
4. Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neurosci Bull 29:603-6135. Schrantee A, Mutsaerts H, Bouziane C, Tamminga H, Bottelier MA, Reneman L (2017)
5. The age-dependent effects of a single-dose methylphenidate challenge on cerebral perfusion in patients with attention-deficit/hyperactivity disorder. Neuroimage Clin 13:123-1296. Tan YW, Liu L, Wang YF et al (2020)
6. Alterations of cerebral perfusion and functional brain connectivity in medication-naïve male adults with attention-deficit/hyperactivity disorder. CNS Neurosci Ther 26:197-2067. Wu J, Xiao H, Sun H, Zou L, Zhu LQ (2012)
7. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol 45:605-6208. Gao Y, Shuai D, Bu X et al (2019)
8. Impairments of large-scale functional networks in attention-deficit/hyperactivity disorder: a meta-analysis of resting-state functional connectivity. Psychol Med 49:2475-2485
Figures