3605
Dysfunctional interaction between the dorsal attention network and the default mode network in Patients with Type 2 Diabetes Mellitus1Department of MRI, Shaanxi Provincial People’s Hospital, Xi’an, China, 2Philips Healthcare, Xi'an, China, 3Xi'an Medical University, Xi’an, China
Synopsis
To investigate the specific neural substrate of type 2 diabetes mellitus (T2DM) -related cognitive impairment, independent component analysis (ICA) and functional network connection analysis (FNC) methods were applied to resting-state fMRI images from 44 patients with T2DM and 47 healthy controls (HCs). This study found the inherent neural mechanism between the dorsal attention network (DAN) and default mode network (DMN) was eliminated, and these abnormal changes were correlated with the scores of multiple neuropsychological assessments (P < 0.05), indicated that the abnormal changes of the DAN-DMN may be the neural basis of T2DM-related cognitive deficits.
Introduction
The risk of cognitive impairment in patients with type 2 diabetes mellitus (T2DM) is significantly higher than that in the general population1, but the exact neurophysiological mechanism underlying this is still unclear. An abnormal change in the intrinsic anticorrelation of the dorsal attention network (DAN) and default mode network (DMN) is thought to be the mechanism underlying cognitive deficits that occur in many psychiatric disorders2, 3, but this association has rarely been tested in T2DM. Independent component analysis (ICA) and functional network connection analysis (FNC) methods can automatically identify meaningful brain networks and directly measure the interactions within and between multiple brain networks. Therefore, ICA and FNC methods were used in this study to explore the function network connectivity of their interaction within and between DAN and DMN in patients with T2DM.Material and Methods
MRI data were obtained from 44 T2DM patients and 47 sex-, age-, and education-matched healthy controls (HCs) on a 3.0-Tesla scanner (Ingenia, Philips healthcare, the Nertherlands) using a 16-channel phased-array head coil. Sagittal 3-dimensional T1-weighted images were acquired with the following parameters: TR = 7.5 ms, TE = 3.5 ms, FA = 8°, FOV = 250 mm × 250 mm, matrix = 256 × 256, slice thickness = 1 mm, no gap, and 328 sagittal slices. Resting-state functional BOLD images were obtained by using a gradient-echo planar sequence with the following parameters: TR = 2000 ms, TE = 30 ms, slices =34, thickness = 4 mm, gap =0 mm, FOV = 230 mm × 230 mm, matrix = 128 × 128, FA = 90°, and 200 volumes. The clinical data and a battery of neuropsychological tests were also assessed (Table 1). GRETNA (https://www.nitrc.org/projects/gretna/) was used to preprocess the functional image data. Two-sample t-test was utilized to compare the clinical features, neuropsychological scores, the functional connectivity (FC) of the resting-state networks (RSNs) of their interaction within and between DAN and DMN between two groups (for FDR correction, P < 0.05).Results
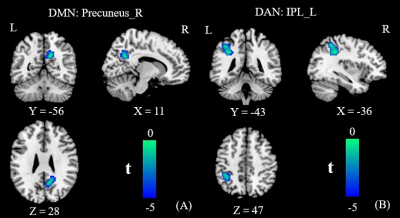
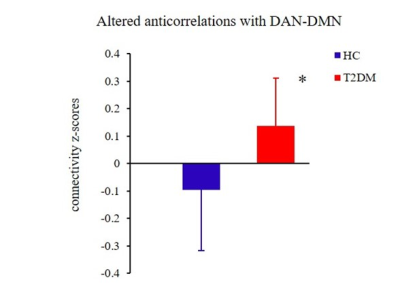
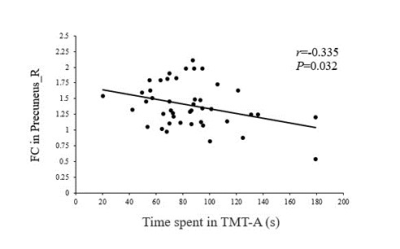
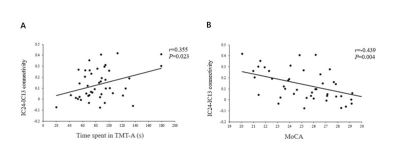
The patients exhibited significantly higher levels of HbA1c and FBG than the HCs did (all Ps < 0.01). The patients also performed significantly worse on the neuropsychological assessments: the MoCA (P < 0.01) and the TMT-A (P < 0.05). Relative to the HCs, the T2DM patients had decreased FC in the right precuneus within the DMN and decreased FC in the left inferior parietal lobule (IPL) within the DAN (Figure 1). Subsequent FNC analysis showed that the patients with T2DM displayed significantly increased inter-network connectivity than HCs between the DAN and DMN; specifically, IC24 and IC13 (Figure 2). The FC of the right precuneus within the DMN was inversely correlated with TMT-A scores (r = -0.335, P = 0.032) in patients with T2DM (Figure 3), and the connectivity strength between the IC24 and IC13 in the DAN and DMN was significantly correlated with the TMT-A scores (r = 0.355, P = 0.023) and the MoCA scores (r = -0.439, P = 0.004) (Figure 4).Discussion
These findings suggest that the FC changes of the DMN and DAN are mainly characterized by a loss of intra-network connectivity and an abnormally increased inter-network connectivity. The precuneus-DMN is one of the brain regions with the highest spontaneous neural activity and metabolism in the resting-state4. Insulin resistance and hyperglycemia could accelerate β-amyloid deposition5, which may be the reason for the decreased FC of the precuneus in the current study. The IPL-DAN is involved in the regulation and integration of attention6. Clinical and epidemiological studies have shown that attention is compromised in patients with T2DM7. Considering the central role of the IPL in the DAN, we speculate that abnormal changes in the IPL may be the neural basis of attentional impairment in T2DM. The anticorrelations of the DAN-DMN might be interpreted as competition between focused attention and processes subserving stimulus independent thought, which indicates that the networks’ intrinsic functional antagonism supports a range of cognitive functions2. And our study found patients with T2DM seem to attenuate this decoupling effect, the reduced anticorrelation between the DAN and DMN is thought to be related to the poor modulation of attentional processes in response to shifting cognitive demands and inefficiency in processing cognitive resources. This is consistent with the clinical observations that T2DM patients have a significantly prolonged response time to stimulus signals, diminished ability to focus attention, and other cognitive impairments8. Furthermore, the strength of connectivity between the DAN and DMN core subsystem in patients with T2DM were negatively correlated with their MoCA scores, and positively correlated with their TMT-A scores. This further illustrates that an abnormal network interaction between the DAN and DMN may be the neural basis of attention and general cognitive dysfunction in T2DM.Conclusion
In summary, this study not only confirmed the dysfunction within the DAN and DMN in T2DM patients, but also discovered that (a) the inherent neural mechanism between DAN and DMN was eliminated, and that (b) the abnormal changes in FC between the two networks were related to attention and general cognitive function. These findings indicate that abnormal DAN-DMN interactions may be the neural basis of T2DM-related cognitive deficits.Acknowledgements
This research was supported by the National Natural Science Foundation of China (81270416), the Key Research and Development Program of Shaanxi Province of China (2018ZDXM-SF-038), the Social Development Science and Technology Research Project of Shaanxi Province of China (2019SF-131), and the Shaanxi Provincial People’s Hospital Technological Development Incubation Foundation of China (2020YXM-04).References
1. Karvani M, Simos P, Stavrakaki S, et al. Neurocognitive impairment in type 2 diabetes mellitus[J]. Hormones (Athens), 2019, 18 (4): 523-534.
2. Wang J, Liu J, Wang Z, et al. Dysfunctional interactions between the default mode network and the dorsal attention network in subtypes of amnestic mild cognitive impairment[J]. Aging (Albany NY), 2019, 11 (20): 9147-9166.
3. Song Z, Chen J, Wen Z, et al. Abnormal functional connectivity and effective connectivity between the default mode network and attention networks in patients with alcohol-use disorder[J]. Acta Radiol, 2021, 62 (2): 251-259.
4. Tessitore A, Esposito F, Vitale C, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease[J]. Neurology, 2012, 79 (23): 2226-2232.
5. Biessels GJ, De Leeuw FE, Lindeboom J, et al. Increased cortical atrophy in patients with Alzheimer's disease and type 2 diabetes mellitus[J]. J Neurol Neurosurg Psychiatry, 2006, 77 (3): 304-307.
6. Clemens B, Zvyagintsev M, Sack AT, et al. Comparison of fMRI activation patterns for test and training procedures of alertness and focused attention[J]. Restor Neurol Neurosci, 2013, 31 (3): 311-336.
7. Garcia-Casares N, Jorge RE, Garcia-Arnes JA, et al. Cognitive dysfunctions in middle-aged type 2 diabetic patients and neuroimaging correlations: a cross-sectional study[J]. J Alzheimers Dis, 2014, 42 (4): 1337-1346.
8. Cooray G, Nilsson E, Wahlin A, et al. Effects of intensified metabolic control on CNS function in type 2 diabetes[J]. Psychoneuroendocrinology, 2011, 36 (1): 77-86.
Figures