3602
Transcriptomic Signatures Associated With Gray Matter Volume Changes In Patients With Functional Constipation1Shanghai Tenth People’s Hospital, Shanghai, China, 2Philips Healthcare, Shanghai, China
Synopsis
Functional constipation (FCon) is a common functional gastrointestinal disorder, with a considerable proportion of patients has anxiety and depression. Neuroimaging studies have shown that the functional/structural abnormalities across patients. We aimed to better understand the relationships between cortical atrophy and clinical observations in FCon, and its relationship with the underlying molecular mechanisms. Based on the densely sampled gene expression data from the Allen Human Brain Atlas, we conducted the transcription-neuroimaging association analysis and found genes associated with the central nervous system and the bowel to understand the molecular functions of brain regions that are vulnerable to cortical atrophy in FCon.
Introduction
Functional constipation (FCon), which belongs to the functional gastrointestinal disorder (FGID), is a common disease and significantly impacts daily life1-3. FGID patients have been progressively proven with functional and structural alterations in various brain regions4-6, but whether and how FCon affects the brain gray matter volume (GMV) remains unclear; besides which genes are associated with the GMV changes in FCon is largely unknown. In the current study, based on the densely sampled gene expression data of six post-mortem brains from the Allen Human Brain Atlas,we conducted the transcription-neuroimaging association analysis to test which genes are associated with GMV changes in FCon to better understand the molecular functions of brain regions that are vulnerable to cortical atrophy in FCon.Methods
Thirty patients with FCon (10 males, right-handed, 46.00 ± 18.03 years) were enrolled into the study. The healthy control group comprised thirty subjects with age and gender-matched. A series of clinical assessment forms were displayed to all members 7-9. Sagittal 3D high-resolution T1-weighted data were collected. All the structural MRI data were preprocessed utilizing CAT12 software to acquire the normalized, modulated, and smoothed GMV images and each voxel represented volume information. Voxel-based comparisons were conducted to identify the brain regions with significant group differences in GMV. Gene expression data were acquired from the AHBA10.These data were processed using a new pipeline that was proposed to link whole-brain gene expression profiles to neuroimaging data11. There were 820 samples (the first dataset) in the two donated brains, and 1782 samples (the second dataset) in the six donated brains only with the left-brain gene expression data. Finally, we gained normalized expression data of 10,185 genes for each tissue sample. Based on the expression values of the 10185 genes from two AHBA datasets and the two sets of case-control GMV differences (t-statistic values), cross-sample (n = 10185) non-parametric Spearman rank was performed to determine the correlations between gene expression and GMV changes in FCon patients separately. The number of comparisons (n = 10185) at the gene level was further corrected with a significance threshold of P <2.55×10−4 = 0.05/10185 (Bonferroni correction). Finally, genes associated with GMV alterations in FCon patients were defined as those whose expression values were significantly correlated with case-control GMV differences derived from two expression datasets.Result
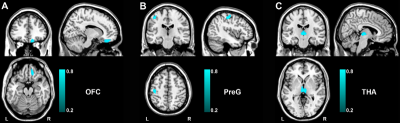
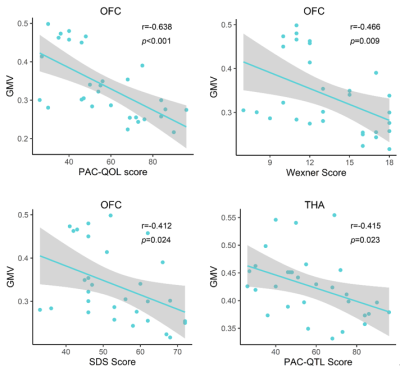
Compared to HC groups, we found that FCon patients primarily demonstrated decreased GMV in the right orbital prefrontal cortex(OFC),left precentral gyrus(PreG) and bilateral thalamus(THA). (Fig1).Correlation analysis showed that the PAC-QOL, Wexner constipation score and SDS score were negatively correlated with GMV in the OFC (Fig.2). There were negative correlations between PAC-QOL score and GMV in the bilateral THA (r = −0.415, P = 0.023) (Fig.2). A cross-sample spatial correlation was performed between gene expression and GMV changes in FCon patients. Based on case-control GMV differences, 345 genes were found to have significant association with GMV alterations in FCon patients in the first dataset, and 208 genes in the second dataset (P < 0.05, Bonferroni corrected). We finally selected the overlapped 18 genes of the two independent gene expression datasets.Discussion
In this study,we found 18 genes’ expression values showed robust correlations with GMV changes in FCon, including the orbitofrontal cortex, precentral gyrus and thalamus. These outcomes could highlight our recognition of the transcriptional features correlated with GMV changes in FCon patients. Here, we found the decreased GMV in the right OFC and its association with constipation scores and depression. As one of the least understood regions, OFC assumes a vital part in emotional regulation. Lots of previous studies support the vital role of OFC in regulating visceral function 12-14. Meanwhile, increased baseline activity in OFC was found in patients with FCon and showed a correlation with the sensation of incomplete evacuation15. In this study, we found the decreased GMV in the right OFC showed association with constipation scores and depression, indicating that the structural injury in OFC might cause the functional abnormality in visceral sensory and motor integration and emotional processing. In addition, we noticed reduced GMV in the left precentral gyrus and the bilateral thalamus, and the GMV of bilateral thalamus in patients with FCon showed association with PAC-QOL score. The precentral gyrus contributes to controlling the movement execution15. The structural abnormality of PreG in patients with FCon indicated the altered ability to control bowel movement 5. Thalamus, as an integrative hub, prominently participates in relaying/integrating/transmitting numerous inputs and connections with various cortical brain areas 16,17. Currently, we identified that the expression of genes (VWA3A, ZBBX, PTPRT, ASB2, PXYLP1, STAC2, ZNF385D, SMCO4, PTGIS, SATB2, HACL1, CSDC2, PCNT and FOSB) showed positive correlations with GMV difference, and the expression of genes (ONECUT1, PDZD2, SLC15A3 and SLC17A6) showed negative correlations. Even though the genes related to GMV changes in FCon is a backhanded technique, we accept that the strategy can suggest valuable discovery on account of such firsthand datasets lacking18.Conclution
we performed transcriptional neuroimaging association to define the genes appearing correlation with GMV changes in FCon. The identified 18 genes, accordantly manifesting prominent relationships between GMV alterations in FCon and gene expression value, could be regarded as candidates to detect biological mechanisms of abnormality in FCon patients.Acknowledgements
Funding This study was funded by the National Natural Science Foundation of China (No 81871325).
References
1.Bongers ME, van Dijk M, Benninga MA, Grootenhuis MA. Health related quality of life in children with constipation-associated fecal incontinencec..J Pediatr;2009;154(5):749-53.
2.Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of Functional Constipation in Children: Therapy in Practice. Paediatr Drugs;2015;17(5):349-60.
3. Hosseinzadeh ST, Poorsaadati S, Radkani B, Forootan M. Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench ;2011;4(3):159-63.
4. Duan S, Liu L, Li G, Wang J, Hu Y, Zhang W, et al. Altered Functional Connectivity Within and Between Salience and Sensorimotor Networks in Patients With Functional Constipation. Front Neurosci ;2021; 15:628880.
5. Hu C, Liu L, Liu L, Zhang J, Hu Y, Zhang W, et al. Cortical morphometry alterations in brain regions involved in emotional, motor-control and self-referential processing in patients with functional constipation. Brain Imaging Behav;2020;14(5):1899-1907.
6. Jin Q, Duan S, Li G, Sun L, Hu Y, Hu C, et al. Sex-related differences in resting-state brain activity and connectivity in the orbital frontal cortex and insula in patients with functional constipation. Neurogastroenterol Motil ;2019;31(5):e13566.
7. Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol ;2005;40(5):540-51.
8. Zung WW. A Self-Rating Depression Scale. Arch Gen Psychiatry;1965;12:63-70.
9. Zung WW. A rating instrument for anxiety disorders. Psychosomatics;1971;12(6):371-9.
10. Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature;2012;489(7416):391-399.
11. Arnatkeviciute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage ;2019; 189:353-367.
12. Bongers ME, van Dijk M, Benninga MA, Grootenhuis MA. Health related quality of life in children with constipation-associated fecal incontinence. J Pediatr;2009;154(5):749-53.
13. Rolls ET. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia ;2019;128:14-43.
14. Guleria A, Karyampudi A, Singh R, Khetrapal CL, Verma A, Ghoshal UC, et al. Mapping of Brain Activations to Rectal Balloon Distension Stimuli in Male Patients with Irritable Bowel Syndrome Using Functional Magnetic Resonance Imaging. J Neurogastroenterol Motil;2017;23(3):415-427.
15. Zhu Q, Cai W, Zheng J, Li G, Meng Q, Liu Q, et al. Distinct resting-state brain activity in patients with functional constipation. Neurosci Lett;2016;632:141-6.
16. Rouiller EM, Tanne J, Moret V, Boussaoud D. Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. J Comp Neurol ;1999; 409(1):131-52.
17.Sherman SM. Functioning of Circuits Connecting Thalamus and Cortex. Compr Physiol ;2017;7(2):713-739.
18.Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science;2015;348(6240):1241-4.
Figures