3597
PM2.5 exposure induces structural and functional changes in the mouse brain1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China
Synopsis
We investigated the structural and functional changes induced by exposure in PM2.5 in the mouse model using MRI at 11.7T. We compared the gray matter volume ratio (GMR) of major brain regions between mice exposed to concentrated PM2.5 (PM) and mice exposed to filtered air (FA) after 30-day and 90-day exposure. Our results demonstrate that exposure to PM2.5 for 90 days may induce brain structural and functional changes.
Introduction
Fine particulate matter with an aerodynamic equivalent diameter ≤ 2.5 microns or less, named PM2.5, is the main component of air pollution. PM2.5 exposure has greatly threatened human health through the respiratory. Due to the absence of well-designed normal controls, PM2.5 studies on humans still have some limitations. Therefore, it is important to perform laboratory investigations to understand the consequence of PM2.5 exposure on brain functions. Several publications on animal models, i.e. mouse under exposure to concentrated PM2.5 compared with filtered air have been conducted and found that PM2.5 is associated with the development of cardiopulmonary diseases, diabetes, and mental disorders by affecting inflammation1–4, oxidative stress5, and neurotransmitters6. However, the neuroimaging changes induced by PM2.5 exposure in the mouse brain have not been fully elucidated. By taking advantage of ultra-high magnetic fields, in the present work, we aimed to investigate whether PM2.5 exposure induces structural and functional changes in the mouse brain at 11.7T.Materials and Methods
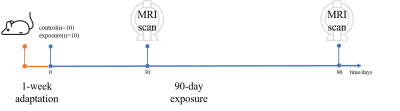
Animal model. A total of 20 C57BL/6J mice (male, 8 weeks old) were obtained from Slac Laboratory (Shanghai, China). All mice were adaptive feeding for 1 week. All experimental protocols were approved by the Animal Ethics Committee of Fudan University.Exposure to PM2.5. As shown in Figure 1, after 1-week adaptation feeding, the mice were divided into two groups (FA, exposure to filtered air; PM exposure to concentrated PM2.5). The FA mice and PM mice were respectively exposed to FA or concentrated PM2.5 in “Shanghai Meteorological and Environmental Animal Exposure System (Shanghai-METAS)”. The “Shanghai-METAS” is located in the School of Public Health at Fudan University at Xujiahui District in Shanghai where most of ambient PM2.5 is attributed to traffic exhausts. The concentrated PM2.5 was generated using a versatile aerosol concentration enrichment system (VACES) that has been published previously.7–9 The duration of the exposure was 8 h per day, 6 days per week (except Sunday) for 90 days. The real-time concentrations of PM2.5 were continuously measured by TEOM 1405 (Thermo, USA), and simultaneously followed by sampling the PM2.5 on filters to determine the accurate concentrations. During exposure, the average PM2.5 concentrations in the exposure chamber were 13.7μg/m³ for FA and 255.1μg/m³ for PM. MRI was performed on 30 days and 90 days during exposure.
Data Acquisition. MRI experiments were performed at an 11.7T BioSpec 117/16 USR MRI system equipped with a CryoProbe (Bruker BioSpin, Ettlingen, Germany). T2-weighted images of the whole brain were acquired using an FSE sequence with the following parameters: TR/TE, 4500/30 ms; FOV, 16mm×16mm; Slice thickness, 0.4mm; Matrix size, 192 × 192 × 40. Functional MRI (fMRI) was acquired using an EPI sequence with the following parameters: TR/TE, 1500/10 ms; FOV 16mm×16mm; Slice thickness, 0.4mm; Matrix size 100 x 100 x 32; Timepoint 220.
Data analysis. Voxel-based morphometry (VBM) method was used to measure the gray matter volume (GMV) of brain regions based on a mouse atlas10 using T2-weighted images. Gray matter volume ratios (GMR) were calculated by dividing the gray matter volume of the whole brain to exclude the influence of individual differences. Otherwise, mReHo was calculated and analysed using rest-fMRI images with SPM12. For the normal distribution verified by the Shapiro-Wilk test, two-sample t-test and paired t-test were performed for measurements (GMR & mReHo) of different mouse groups on the same scan time and of the same group on different scan times, separately. The statistical analyses of VBM measurements were performed using statsmodel11 in Python with p<0.05 as the significance criterion.
Results and Discussion
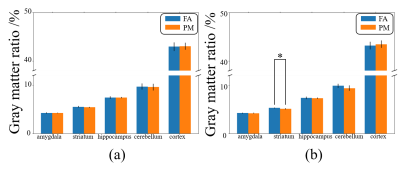
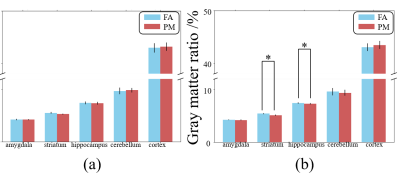
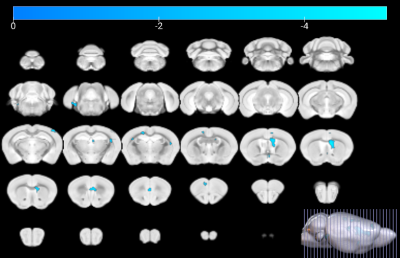
Figure 1 shows a schematic of the experimental setup. As shown in Figure 2, the GMR of main brain regions has no significant differences after 30-day exposure, while the GMR of the striatum in PM (5.09%) is significantly smaller than FA (5.30%) after 90-day exposure. As shown in Figure 3, in the FA group, the GMR has no significant difference between exposure for 30 days and 90 days. By contrast, in the PM group, compared with 90-day exposure, the GMR of striatum and hippocampus are significantly smaller than 30-day by 6.09% and 2.02%, respectively. As shown in Figure 4, compared with the FA group, mReHo of the PM group reduces significantly in the entorhinal and frontal lobe (p < 0.01, cluster size > 10) when the exposure to PM2.5 for 90 days. The longitudinal difference between the FA and PM groups indicates that the influence induced by PM2.5 in the brain may increase with the increase of exposure time.14,15 Our results demonstrate that the brain structural and functional changes can be affected by PM2.5 exposure. The possible reason may be that PM2.5 triggers a pro-inflammatory response in nervous tissue and adversely affects the central nervous system.12,13Conclusion
Our data showed that the GMR of the striatum and hippocampus were significantly smaller after exposure to PM2.5 for 90 days. In addition, the mReHo reduces significantly in the entorhinal and frontal lobe after exposure to PM2.5 for 90 days. These results indicate that PM2.5 exposure may induce brain structural and functional changes in mice.Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81873893), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJLab, and Shanghai Center for Brain Science and Brain-Inspired Technology.References
1. Air Pollution is Associated with the Development of Atherosclerosis Via the Cooperation of CD36 and NLRP3 Inflammasome in ApoE-/- Mice. (2018).
2. Jiang, S. et al. The severity of lung injury and metabolic disorders induced by ambient PM2.5 exposure is associated with cumulative dose. Inhal. Toxicol. 30, 239–246 (2018).
3. Du, X. et al. Fine particulate matter-induced cardiovascular injury is associated with NLRP3 inflammasome activation in Apo E mice. Ecotoxicol. Environ. Saf. 174, 92–99 (2019).
4. Liu, C., Yang, J., Guan, L., Zhu, Y. & Geng, X. Filtered air intervention reduces inflammation and hypothalamus-pituitary-adrenal axis activation in adult male and female rats after PM 2.5 exposure. Environ. Sci. Pollut. Res. Int. 27, 35341–35348 (2020).
5. Sunyer, J. et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 12, e1001792 (2015).
6. Allen, J. L. et al. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 122, 939–945 (2014).
7. Sun, Q. Long-term Air Pollution Exposure and Acceleration of Atherosclerosis and Vascular Inflammation in an Animal Model. JAMA vol. 294 3003 (2005).
8. Sun, Q. et al. Ambient Air Pollution Exaggerates Adipose Inflammation and Insulin Resistance in a Mouse Model of Diet-Induced Obesity. Circulation vol. 119 538–546 (2009).
9. Xu, X. et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. 124, 88–98 (2011).
10. Dorr, A. E., Lerch, J. P., Spring, S., Kabani, N. & Henkelman, R. M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42, 60–69 (2008).
11. Seabold, S. & Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. Proceedings of the 9th Python in Science Conference (2010) doi:10.25080/majora-92bf1922-011.
12. Campbell, A. et al. Particulate Matter in Polluted Air May Increase Biomarkers of Inflammation in Mouse Brain. NeuroToxicology vol. 26 133–140 (2005).
13. Fonken, L. K. et al. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 16, 987–95, 973 (2011).
14. Calderón-Garcidueñas, L. et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 68, 117–127 (2008).
15. Calderón-Garcidueñas, L. et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 77, 345–355 (2011).
Figures