3594
Longitudinal Development of Regional Cerebellar Volumes from Birth to 27 Months of Age1National Key Discipline of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China, 2Department of Radiology and Biomedical Research Imaging Center (BRIC), University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Early cerebellar development in infant brains is very dynamic and highly related to normal cognitive functions and neurodevelopmental disorders, but remains largely unexplored, due to the lack of densely-sampled longitudinal data of early ages. Herein, we unprecedently explored the dynamic developmental trajectories of the volumes in 27 cerebellar lobules based on 511 high-resolution longitudinal structural MRI scans from 235 healthy infants from the Baby Connectome Project (BCP) densely covering the age ranging from birth to 27 months. The trajectories of the cerebellar structures reveal lobule-specific nonlinear developmental patterns and are sexually dimorphic starting from different ages.
Introduction
The cerebellum plays a prominent role in many functions beside somatomotor control, according to recent functional MRI studies1. Moreover, different age ranges show different region-specific developmental trajectories of cerebellar lobules and functional activations also have been found in different lobules during different cognitive and motor tasks2-5. However, due to the limited availability of longitudinal data and image processing methods, few studies have characterized regional cerebellar growth trajectories at early ages, during which the cerebellum undergoes dramatic, nonuniform, and nonlinear changes. This study aims to fill this critical gap by exploring the volumetric developmental trajectories of 27 cerebellar lobules based on 511 high-resolution longitudinal structural MRI scans from 235 healthy subjects densely covering the age ranging from birth to 27 months.Materials and Methods
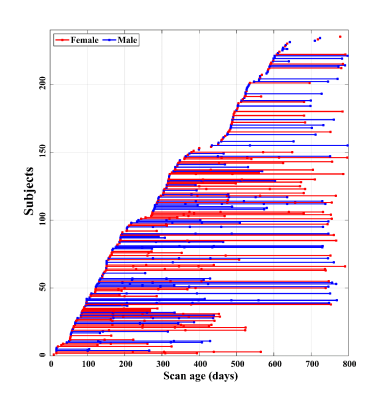
In total, 511 longitudinal structural MRI scans, including T1-weighted and T2-weighted images, from 235 cognitively normal, term born subjects (113 males/122 females) from birth to 27 months of age were included, which were collected by the UNC/UMN Baby Connectome Project (BCP)6. The scan age and gender distributions were showed in Fig. 1. We first preprocessed the MR images and extracted the cerebellum using the iBEAT V2.0 Cloud7. The cerebellar parcellation maps were obtained by longitudinally propagating the SUIT parcellation8 to each scan. Specifically, we first built a 4D infant cerebellum atlas with dense time points (i.e., 0, 3, 6, 9, 12, 18, and 24 months) by using the state-of-the-art SyGN template construction technique9 from ANTs10. Then the 4D infant cerebellum atlas11 was leveraged as a bridge in an age decreasing manner to obtain the parcellation maps, which were further manually edited using ITK-snap12. To ensure the within-subject spatial-temporal consistency of parcellations, for longitudinal scans, image registration was performed successively between each two adjacent longitudinal scans in an age decreasing manner as well. Thus, the whole cerebellar image was parcellated into 27 sub-regions: bilateral lobules I-IV, V, VI, Crus I, Crus II, VII B, VIIIA, VIIIB, IX and X; Vermis VI, Crus II, VII B, VIII A, VIII B, IX and X. Finally, the volumes of the 27 sub-regions were calculated. The non-parametric generalized additive mixed model (GAMM) was employed to fit the population’s volumetric trajectories of each sub-region, which is more suitable for handling dynamic and complex data-driven changes13.Results and Discussion
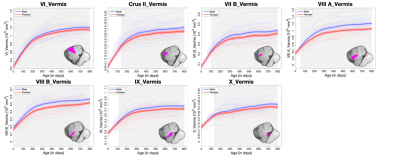
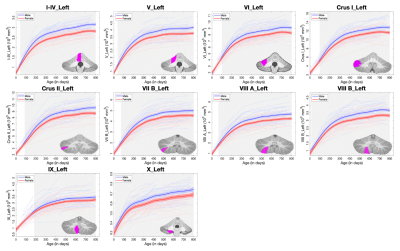
The longitudinal trajectory of each subject and the GAMM-fitted population’s curves by gender are depicted for vermis volumes (Fig. 2) and lobular volumes in the left hemisphere (Fig. 3). The volume of each structure shows a rapid increase at first followed by a relative sluggish growth thereafter. Specifically, the volumetric growth rates of most structures in vermis decrease at around 300 days, later than that of vermis X (decreasing within 200 days). Additionally, vermis X exhibited a relatively small growth rate (116% during the first 12 months) than other structures in vermis. The lobules also exhibited similar trend, and several lobules rapidly increased until 12 months after birth, such as Crus I and VII B. Structures with the largest growth rates during the first 12 months include: 1) VI, Crus I, and VII B (387%/444%/339%) in males; 2) Crus I, VII B, and VIII A (335%/324%/326%) in females. However, lobule X exhibited relatively small growth rates with 108% in males and 111% in females during the first year. Besides, the developmental trajectories of the cerebellar sub-regions are sexually dimorphic, and each sub-region exhibits a larger absolute volume in males than females emerging from different ages. For example, a significant gender difference of the absolute volume in lobule IX appeared at around 200 days, later than that of most lobules, while several lobules exhibited gender differences earlier such as VIII B (at around 10 days after birth). These lobule-specific early growth trajectories may provide important insights into the basis of cerebellar function development.Conclusion
The early volumetric developmental trajectories of the cerebellar structures reveal lobule-specific nonlinear growth patterns with rapid increases at first followed by relative sluggish growth thereafter and are sexually dimorphic starting from different ages. This discovery is an important reference for understanding both normal and abnormal cerebellar development.Acknowledgements
This work was partially supported by NIH grants (MH116225, MH117943, MH104324, MH109773, and MH123202). This work also utilizes approaches developed by an NIH grant (1U01MH110274) and the efforts of the UNC/UMN Baby Connectome Project Consortium.References
1. Buckner RL, Krienen FM, Castellanos A, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106(5):2322-2345.
2. Han S, An Y, Carass A, et al. Longitudinal analysis of regional cerebellum volumes during normal aging. Neuroimage. 2020;220:117062.
3. Tiemeier H, Lenroot RK, Greenstein DK, et al. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49(1):63-70.
4. Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176-12182.
5. Guell X, Gabrieli JDE, Schmahmann JD. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. 2018;172:437-449.
6. Howell BR, Styner MA, Gao W, et al. The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. Neuroimage. 2019;185:891-905.
7. Wang L, Li G, Shi F, et al. Volume-based analysis of 6-month-old infant brain MRI for autism biomarker identification and early diagnosis. International conference on medical image computing and computer-assisted intervention. 2018;Springer:411-419.
8. Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39-46.
9. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
10. Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight j. 2009;2(365):1-35.
11. Chen L, Wu Z, Hu D, et al. Construction of Longitudinally Consistent 4D Infant Cerebellum Atlases Based on Deep Learning. International Conference on Medical Image Computing and Computer-Assisted Intervention. 2021; Springer: 139-149.
12. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128.
13. Wang F, Lian C, Wu Z, et al. Developmental topography of cortical thickness during infancy. Proc Natl Acad Sci. 2019;116(32):15855-15860.
Figures