3593
Postnatal experience promotes the thickening but not myelination of the neonatal ventral visual cortex1College of Biomedical Engineering & Instrument Science, Zhejiang University, Zhejiang, China, 2MR Collaboration, Siemens Healthineers Ltd, Shanghai, China
Synopsis
Postnatal experience is important for the development of the visual cortex in animal newborns, but its influence in human infants remains unclear. We collected a dataset from the developing human connectome project, including multi-modal MRI of 783 newborns. We found the cortical thickness (CT) and myelination of ventral visual cortex were significantly increased between 37 and 45 weeks of postmenstrual menstrual age (PMA). Interestingly, the CT but not myelination showed significant correlation with postnatal experience in both the V1 and higher-level visual cortex. Our result suggested that experience plays an important role in early cortical thickening
Introduction
The role of experience in cortical development is controversial in neuroscientific research 1,2. Given its ecological importance across species, vision has long been taken as a representative modality to investigate this question 3–6. Although many studies have assessed the complicated interaction between postnatal experience and cortical development of the visual cortex in animals 3,4,7, the influence of postnatal experience on the development of the visual cortex of human infants remains unclear. To investigate this question, we collected the dataset from the developing human connectome project (dHCP, www.developingconnectome.org), comprising multi-modal MRI of 783 newborns. We focused on the ventral visual cortex in these newborns, and characterized the developmental trajectory of cortical morphology (cortical thickness, CT) and microstructure (myelination) with respect to postmenstrual age (PMA) and postnatal experience, namely, the interval between gestational age at birth (GA) and PMA.Method
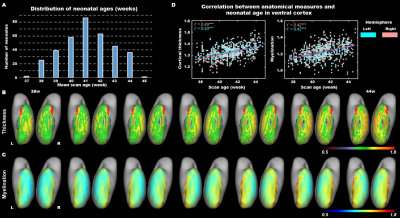
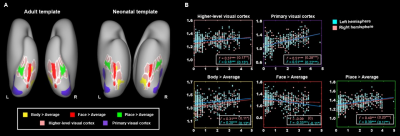
The present study included 407 neonates after data preprocessing, 355 of them were born at term. T2-weighted and inversion recovery T1-weighted multi-slice fast spin-echo images were acquired in sagittal and axial stacks with resolution of 0.8 × 0.8 × 1.6 mm3. After basic preprocessing following dHCP pipeline 8, we registered the individual cortex to the dHCP symmetric template 9 using multimodal surface matching 10, and extracted the cortical measurements within the ventral cortex, which was empirically predefined as the 34 ventral patches in HCP-MMP atlas 11 in adults and projected into neonatal space (Fig. 1). We accessed the postnatal effect based on Pearson correlation and partial correlation between postnatal time and cortical measurements. We focused on both the primary visual cortex (V1) and higher-level visual cortex (ventral occipital temporal cortex, VOTC) that were further segmented into three category-selective domains (place/body/face area), by transforming the domain definition from adult space 12–15 into the neonatal space (Fig 3A).Results
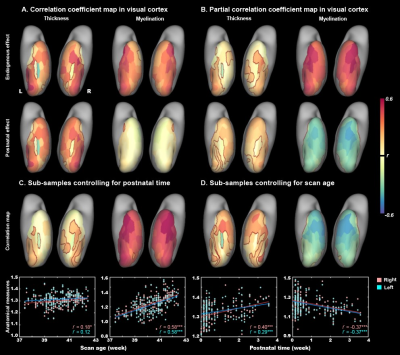
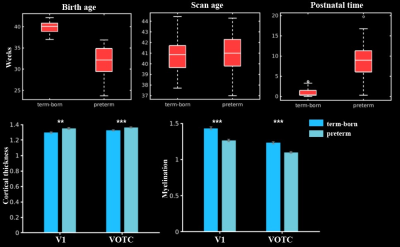
In general, the CT and myelination of the ventral cortex were significantly increased between 37 and 45 weeks of PMA (rs = 0.42 – 0.49; ps < 10-9; Fig 1). Taking the 34 patches as ROIs, we found distinct spatial differences in the development within the ventral cortex, where the posterior and medial areas showed a higher PMA-CT correlation compared to anterior and lateral areas, but the highest PMA-myelination correlation was in the anterior area (Fig 2A). The postnatal time showed a significant correlation with CT but not with myelination in the ventral cortex, either with or without regressing PMA (Fig 2A, 2B). We further designed experiments on two sub-samples to separate effects from postnatal experience and PMA: the first sun-sample with a fixed postnatal time (3 days after birth) and the second subset with a fixed PMA within 40 to 42 weeks. In the first sub-samples, the myelination (r = 0.58 and 0.58 for the right and left hemispheres, respectively; ps < 10-9) but not the CT (r = 0.18 and 0.12, p < 0.02 and > 0.10; Fig 2C) of ventral cortex showed strong correlation with PMA. And in the second sub-samples, we found significantly positive CT-PMA correlation (r = 0.40 and 0.29, ps < 0.001) but negative myelination-PMA correlation in ventral cortex (r = -0.37 and -0.37, ps < 10-6; Fig 2D). In addition to the primary visual cortex, the CT of the VOTC was also modulated by the postnatal time (rs = 0.37 and 0.18; ps < 10-3). Specifically, the correlation between postnatal time and CT was significantly positive in the medial part (precursor place area; r = 0.40 and 0.39; ps < 10-9) and the posterior-lateral area (precursor body area; r = 0.33 and 0.24; ps < 10-3) but not in the middle part (precursor face area; rright = -0.09, p > 0.05 and rleft = -0.33, p < 10-9) of the higher-level visual cortex (Fig 3B). Relative to the term-born infants, preterm-born infants have longer postnatal experience but was immature at birth, which made them a good model to compare the influence between early experience and endogenous coding on cortical development. Two sample t-test between the two groups showed at equivalent PMA, the term-born infants had higher myelination (t = 6.83 and 7.19; p < 10-8) but lower CT (t = -3.34 and -3.39; p < 0.002) in both the V1 and VOTC, supporting the previous finding that early thickening of CT in neonates could be modulated by postnatal time but cortical myelination was heavily depending on the GA.Discussion and Conclusion
The present study revealed that the development of the ventral visual cortex is modulated by the postnatal experience of human infants, even in the weeks soon after birth. CT is influenced by synaptogenesis and synaptic pruning, which is highly related to the postnatal experience 16–18. Previous studies have suggested that the initial thickening of the visual cortex did not depend on visual experience 18. Our study further elucidated this processing by separating the postnatal and endogenous effect on cortical development, in which the postnatal experience could accelerate the thickening of the neonatal visual cortex, while myelination appeared to be an independent process that are not directly related to environmental stimuli within the first few days of life.Acknowledgements
Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600), National Natural Science Foundation of China (61801424, 81971606, 82122032), and Science and Technology Department of Zhejiang Province (202006140).References
1. Barlow, H. B. Visual experience and cortical development. Nature 258, 199–204 (1975).
2. White, L. E. & Fitzpatrick, D. Vision and cortical map development. Neuron 56, 327–338 (2007).
3. Crair, M. C., Gillespie, D. C. & Stryker, M. P. The role of visual experience in the development of columns in cat visual cortex. Science. 279, 566–570 (1998).
4. Roy, A., Wang, S., Meschede-Krasa, B., Breffle, J. & Van Hooser, S. D. An early phase of instructive plasticity before the typical onset of sensory experience. Nat. Commun. 11, 1–15 (2020).
5. Li, Y., Van Hooser, S. D., Mazurek, M., White, L. E. & Fitzpatrick, D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature 456, 952–956 (2008).
6. Li, Y., Fitzpatrick, D. & White, L. E. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat. Neurosci. 9, 676–681 (2006).
7. Akerman, C. J., Smyth, D. & Thompson, I. D. Visual Experience before Eye-Opening and the Development of the Retinogeniculate Pathway. Neuron 36, 869–879 (2002).
8. Makropoulos, A. et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173, 88–112 (2018).
9. Bozek, J. et al. Construction of a neonatal cortical surface atlas using Multimodal Surface Matching in the Developing Human Connectome Project. Neuroimage 179, 11–29 (2018).
10. Robinson, E. C. et al. Multimodal surface matching with higher-order smoothness constraints. Neuroimage 167, 453–465 (2018).
11. Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016).
12. Epstein Russell & Kanwisher, N. A cortical representationof the local visual environment. Nature 392, 598–601 (1998).
13. Kanwisher, N., McDermott, J. & Chun, M. M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 17, 4302–4311 (1998).
14. Downing, P. E., Jiang, Y., Shuman, M. & Kanwisher, N. A cortical area selective for visual processing of the human body. Science. 293, 2470–2473 (2001).
15. Grill-Spector, K. & Weiner, K. S. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci. 15, 536–548 (2014).
16. Lyall, A. E. et al. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb. Cortex 25, 2204–2212 (2015).
17. Holtmaat, A. & Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 10, 647–658 (2009).
18. Jiang, J. et al. Thick visual cortex in the early blind. J. Neurosci. 29, 2205–2211 (2009).
Figures