3575
MRSaiFE: towards the real-time prediction of SAR in 3T and 7T MR RF coils - a feasibility study with 10 body models1Radiology, Weill Cornell Medicine, New York, NY, United States, 2General Electric Healthcare, Aurora, OH, United States, 3Biomedical Engineering, University of Minnesota, Minneapolis, MN, United States, 4Center for Devices and Radiological Health, U.S. Food and Drug Administration, Silver Spring, MD, United States, 5Radiology, Stanford University, Stanford, CA, United States
Synopsis
Significant RF power deposition in the body causing local specific absorption rate (SAR) in the form of hotspots is an important safety concern at 3T (128 MHz) and, even more so, at 7T (298 MHz). In this work, we expand the proof-of-concept of artificial intelligence based real-time MRI safety prediction software (MRSaiFE) to 10 body models. We show that SAR patterns can be predicted with a mean squared error (MSE) of less than 1% and a structural similarity index of above 90% for 7T brain and above 85% for 3T body MRI.
Introduction
Ultra-high-field (UHF) magnetic resonance imaging (MRI) comes with improved signal-to-noise-ratio (SNR)1, but non-uniform deposition of radiofrequency (RF) power creates a serious safety concern for clinical applications2. High variation of the specific absorption rate (SAR) in tissues, creates local hotspots, thereby limiting 7T MRI achieving its full potential3. Furthermore, parallel transmit (pTx) technology, commonly used at 7T can lead to even stronger hotspots because of potential constructive interference of electric fields2. For this reason, many institutions are required to use a conservative ratio of peak local to global SAR, such as ~20:14, thereby severely limiting the applied transmit power and thus the imaging performance achievable by 7T MR scanners.Most clinical imaging to date has been performed at field strengths of 3T or lower. Recently, two 7T MR scanners have received FDA approval5, 6. A major limitation at 7T is the inconsistency of SAR limits in IEC 60601-2-334. In the Normal Operating Mode (NOM) the SAR for volume RF transmit coils is limited to 2 W/kg without limiting the local SAR. Local RF transmit coils are limited in the NOM to 10 W/kg averaged over 10g. However, many computational studies have shown that volume RF transmit coils in the NOM can produce a local SAR of greater than 50 W/kg7,8. This factor of >5 inconsistency poses a significant disadvantage for local RF transmit coils.
In this work, we expand the MRSaiFE, an artificial intelligence (AI) based exam-integrated MRI safety prediction software, to demonstrate feasibility in ten body models at 3T and 7T with the goal of conservative SAR estimation with known uncertainty9-12. Using this tool, we hypothesize that estimating SAR-distribution in tissues with an MSE of <1% would be possible.
Methods
A. Data Generation: We used Sim4Life13, and different body models from the Virtual Population14. The anatomical input images were approximated by using gray-scale images of the body models. Image data sets were synthesized as reported before9. Landmark information was encoded in the anatomical images by multiplication with the normalized B1+ field of the unloaded RF coils11.For 3T, a bore-mounted RF body coil (16-rung, high-pass birdcage, 60 cm diameter, 67 cm length), with 4-port circular polarization drive was used. For 7T, a 16-rung birdcage head coil model (30 cm diameter, 25 cm length) was used in quadrature mode. In addition to previous work with two body models, we positioned additional realistic body models14 (Duke, Ella, Charlie, and 3 pregnant women at different gestational stages of 3, 7, and 9 months), at different landmarks reported earlier9. The data were split into three categories for each body model separately with an approximate ratio of 80, 10, and 10% for training, validation, and testing, respectively (see Table 1).
B. Network: 2D U-Net architectures15 were implemented using a cascade of convolutional filters9, 16 paired with nonlinear rectified linear unit (ReLU) activation functions17, batch normalization18, He initialization19, and optimizers of stochastic gradient descent (SGD)20, and Adam21 with default settings9. We compared 3 U-Nets with varying number of contraction (encoder) and expansion (decoder) layers (stages), using different filter feature map lengths (n) and learning rates (LR)9.
C. Testing: Quantitative image quality comparisons were performed between the ground-truth (GT) images (simulated SAR) and the predicted SAR using MSE and structural similarity index (SSIM)22.
Results
Hyperparameter analysis results of the 3 U-Nets are given in Figure 1 for the 3T body coil. Increasing the input stage feature map length improved the SSIM. Training time for the 256-feature map was increased by a factor >2 compared to 128 layers. Among the investigated U-Net architectures, the use of a 4-stage network with 64 feature maps at the initial stage produced the best trade-off.The optimized 4-stage U-Net architecture was analyzed for different body models at different field strengths (Figure 2). For 3T body (top row), an average SSIM of 85.1±6.2% and an average MSE of 0.4±0.4% were observed (Adam). For 7T brain (bottom row), an average SSIM of 90.5±3.6% and an average MSE of 0.7±0.6% (Adam) were observed. The higher spatial resolution of the 7T data (i.e., isotropic pixel size of ~1.33 mm compared to 2.67 mm at 3T) resulted in improved SSIM and MSE.
Predicted hotspots in 10 subjects showed very good agreements between GT and estimation results (Figure 3 and 4). Using the top 15% of max-SAR values of each slice, we obtained a mean SSIM of 97.3%±2.5% demonstrating good agreement on hotspot locations, as well as a relative mean error of 1.5%±1.1% of maximum SAR values when the SAR map was normalized to the mean of the GT. This corresponds to normalizing the predicted SAR map to the, measurable, global SAR at the time of the patient exam.
Conclusion
We expanded the proof of concept for MRSaiFE to ten body models. MRSaiFE predicted SAR maps with a residual MSE of <1% for both 7T head imaging and 3T body imaging. It also achieved an SSIM of >90% for 7T head imaging and >80% for 3T body imaging. We showed that CNNs can predict the SAR distributions at HF and UHF strengths during clinical imaging in real-time by using MRIs and global SAR value as the only inputs.Acknowledgements
This work was supported in part by the National Institute of Health (NIH) through R00EB024341. The authors are also grateful to General Electric Healthcare for their support.
DISCLAIMER: The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or suggested endorsement of such products by the Department of Health and Human Services.
References
1. Springer, E, Dymerska, B, Cardoso, PL, et al. Comparison of Routine Brain Imaging at 3 T and 7 T:. Investigative Radiology 2016;51(8):469-482.
2. Winkler, SA, Rutt, BK. Practical methods for improving B1+ homogeneity in 3 tesla breast imaging. Journal of Magnetic Resonance Imaging 2015;41(4):992-999.
3. Fiedler, TM, Ladd, ME, Bitz, AK. SAR Simulations & Safety. NeuroImage 2018;168:33-58.
4. Commission, IE. IEC 60601-2-33. Medical Electrical Equipment – Part 2-33: Particular Requirements for the Basic Safety and Essential Performance of Magnetic Resonance Equipment for Medical Diagnosis, Edition 3.1. Geneva; 2013.
5. GE-Healthcare. Bringing Ultra-High Field MR Imaging from Research to Clinical: SIGNA 7.0T FDA Cleared; 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K202966.pdf. Accessed November 11 2021.
6. SiemensHealthineers. FDA clears first 7T magnetic resonance imaging device; 2017. https://www.accessdata.fda.gov/cdrh_docs/pdf17/K170840.pdf. Accessed November 11 2021.
7. Murbach M, et al. Virtual population-based assessment of the impact of 3 Tesla radiofrequency shimming and thermoregulation on safety and B1 + uniformity. Magn Reson Med. 2016 Sep; 76(3):986-97.
8. Murbach M, et al. Whole-body and local RF absorption in human models as a function of anatomy and position within 1.5T MR body coil. Magn Reson Med. 2014 Feb;71(2):839-45.
9. Gokyar, S, Robb, FJL, Kainz, W, et al. MRSaiFE: An AI-based Approach Towards the Real-Time Prediction of Specific Absorption Rate. IEEE Access 2021:1-1.
10. Winkler, S, Saniour, I, Chaudhari, A, et al. MRSaiFE: Tissue Heating Prediction for MRI: a Feasibility Study. arXiv pre-print server 2021.
11. Winkler, SA, Motovilova, E, Gokyar, S, et al. MRSaiFE: towards the real-time prediction of tissue heating in MRI - a feasibility study. Proc Intl. Soc. Mag. Reson. Med. 2486. ISMRM; 2021:2486.
12. Winkler, SA, Saniour, I, Robb, F, et al. MRSaiFE: towards the real-time prediction of tissue heating in MRI - a feasibility study. Proc. IEEE IMBioC. Toulouse, France; 2020.
13. https://zmt.swiss/sim4life, Sim4Life, Zurich Med Tech, Zürich, Switzerland. Accessed November 11 2021.
14. https://itis.swiss/virtual-population, IT’IS Foundation, Zürich, Switzerland. Accessed November 11 2021.
15. Ronneberger, O, Fischer, P, Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv pre-print server 2015.
16. Chaudhari, AS, Fang, Z, Kogan, F, et al. Super‐resolution musculoskeletal MRI using deep learning. Magnetic Resonance in Medicine 2018;80(5):2139-2154.
17. Nair, V, Hinton, GE. Rectified linear units improve restricted boltzmann machines. ICML. 2010.
18. Ioffe, S, Szegedy, C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. arXiv pre-print server 2015.
19. He, K, Zhang, X, Ren, S, et al. Delving Deep into Rectifiers: Surpassing Human-Level Performance on ImageNet Classification. arXiv pre-print server 2015.
20. Lecun, Y, Bottou, L, Bengio, Y, et al. Gradient-based learning applied to document recognition. Proceedings of the IEEE 1998;86(11):2278-2324.
21. Kingma, DP, Ba, JL. Adam: A Method for Stochastic Optimization. arXiv pre-print server 2017.
22. Wang, Z, Bovik, AC, Sheikh, HR, et al. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Transactions on Image Processing 2004;13(4):600-612.
Figures
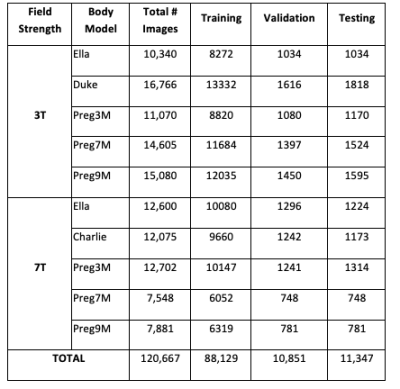
Table 1. Dataset generated in this study. Total number of images for each body model is given different lines along with the number of images used in training, validation, and testing.
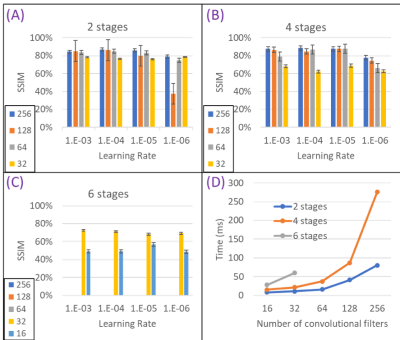
FIGURE 1. Performance comparison of three U-Nets due to different hyperparameters and time budget for Adam. All structures were trained and tested by using Ella at 3T. SSIM of (A) 2-stage, (B) 4-stage, and (C) 6-stage U-Nets are depicted. (D) Total time to train each image (for forward and backward pass) showed an exponential increase among different U-Nets. Using a 4-stage network with 64 feature maps (n) at the initial stage showed the best compromise in terms of SSIM and training times. The complexity of the structure is kept at this level for inter-subject variation and visual analysis.
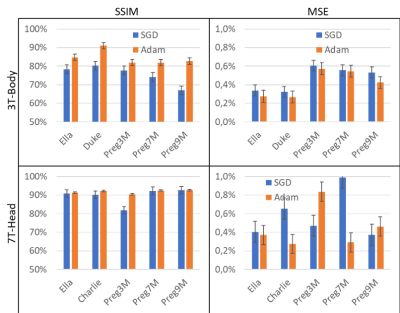
FIGURE 2. Inter-subject performance analysis of the optimal U-Net. Adam optimizer worked better than SGD. (Top Row) For 3T body coil SAR prediction, an average SSIM of 85.1±6.2% and an average MSE of 0.4±0.4 (Adam), and an SSIM of 69.3±4.5% and an MSE of 0.5±0.4% (SGD) were achieved. (Bottom Row) In the 7T, we observed an average SSIM of 90.5±3.6% and an average MSE of 0.7±0.6% (Adam), compared to an SSIM of 81.4±2.6% and an MSE of 0.5±0.5% (SGD). The higher spatial resolution of the 7T data (isotropic pixel size of ~1.33 mm compared to 2.67 mm at 3T) resulted in improved SSIM and MSE.
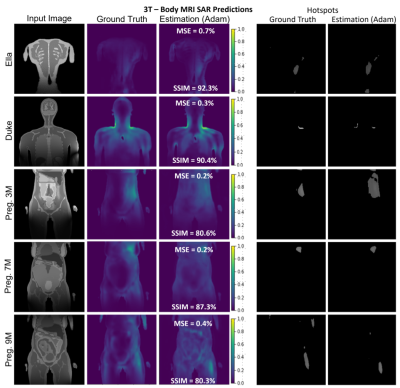
FIGURE 3. A selection of slices for Ella, Duke, and three Pregnant Women of different gestational stages for 3T body imaging. Despite very high variations in the SAR maps and input images, the proposed 4-stage U-Net architecture successfully recovered the distribution with >80% average SSIM and <0.5% average MSE for all body models. Hot spot analysis showed that the proposed architecture may estimate the hot-spot locations and values with a mean SSIM of 97.3%±2.5% depicting good agreement for the hotspot locations, as well as a relative mean error of 1.5%±1.1% for the maximum SAR values.
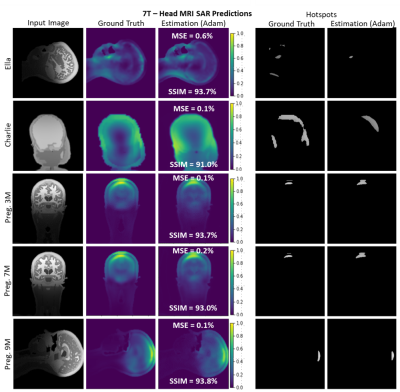
FIGURE 4. A selection of slices for different body models at 7T (Duke is replaced with Charlie in second row). 4-stage U-Net architecture successfully recovers the SAR distribution for all body models (SSIM >91%, MSE<0.5%). It is seen that the spatial information provided by the input images also carried to estimation results without any memorization. Since Ella and the Pregnant Women models provided higher spatial resolution compared to Charlie (second row), estimation performances differ slightly due to encoded spatial information in the input images.