3572
Saturation pre-pulse train optimization for maximization of cucurbit[6]uril hyperpolarized chemical exchange saturation transfer at 3.0 T1Chemistry and Materials Science Program, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Chemistry Department, Lakehead University, Thunder Bay, ON, Canada, 4Applied Life Science Program, Lakehead University, Thunder Bay, ON, Canada, 5Xemed LLC, Durham, NH, United States, 6Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Cucurbit[6]uril (CB6) is a well-known hyperpolarized 129Xe (HP 129Xe) MRI contrast agent. Although in-vivo biodistribution detection of CB6 has already been achieved, no studies have been performed on optimization of the saturation pre-pulse train in order to maximize CB6 performance. In the present work, we demonstrate that utilization of sinusoidal saturation pulses for 129Xe HyperCEST MRI, instead of using conventional three-lobe sinc pulses, yields substantial improvement of CB6 HyperCEST performance and a significant increase in HyperCEST MRI sensitivity. In addition, lower detectable CB6 concentrations are reported for both types of saturation pre-pulses at 3T using a whole-body scanner.
Introduction
Hyperpolarized (HP) chemical exchange saturation transfer (HyperCEST) MRI contrast agents substantially increase the sensitivity of HP 129Xe MRI and allow an advantageous way of conducting molecular imaging while maintaining the spatial resolution of MRI1,2. Although numerous supramolecular cages were utilized for HyperCEST detection3–7, cucurbit[6]uril (CB6) has been demonstrated as a favourable agent for in vivo HyperCEST MRI imaging2,8. Although two studies demonstrated in vivo imaging of CB6 biodistribution in living rats at 3.0T2 and mice at 9.4 T8, none of these studies performed an imaging pulse sequence optimization in order to maximize the observed HyperCEST effect. Furthermore, there has been no deliberate in-depth investigation of the lowest detectable concentration of CB6 with respect to the pulse sequence used. Previous studies used 10 mM2 and 20 mM8 concentrations for in vivo imaging, which might be too high for further clinical translation. In this work, we demonstrated the maximization of the HyperCEST effect in various concentrations of CB6 solutions in PBS by utilizing a sinusoidal saturation radiofrequency (RF) pulse train at the lowest detectability limit of CB6 at 3.0T in a whole-body scanner.Methods
This study was conducted using a clinical Philips Achieva 3.0T MRI scanner. Naturally abundant 129Xe was polarized up to 40% using a Xemed XeBox-10E polarizer (Xemed LCC, USA). Spectral data were acquired in vitro using a glass fritted phantom, which was placed inside of a custom-made quadrature RF coil tuned to the resonance frequency of Xe (35.33 MHz) at 3.0 T and positioned in the center of the bore. Various 2.5mL samples of CB6 (1mM, 0.4mM, 0.25mM, 0.1mM, 25uM, and 10uM) dissolved in PBS (pH 7.4) were drawn into the phantom vessel and HP 129Xe gas was continuously bubbled into solution while HyperCEST depletion spectra were simultaneously acquired. Two different RF saturation pre-pulse trains were used for HyperCEST MRS: 3-lobe sinc (3LS) pulses and sinusoidal pulses. The 3LS saturation pre-pulse train consisted of 16 individual pulses of 30ms duration and a 330° flip angle (FA). The resonance frequency of HP 129Xe dissolved in the solutions was set a 0 ppm. During the HyperCEST experiments, 3LS saturation pulses were applied in a frequency range from -149 ppm to 50 ppm with a step of 3 ppm. The sinusoidal pre-pulse train consisted of 16 sinusoidal RF pulses of 30 ms duration and a 1530° flip angle. Sinusoidal pulses were applied in a range from -150 ppm to 50 ppm with a step of 2 ppm. Dynamic HyperCEST MRS was performed using the following parameters: TR/TE = 10s/0.25ms, receiver bandwidth (BW) = 32kHz, 90o excitation rectangular pulse, 2048 sampling data points. Raw data was initially processed in MATLAB 2021b (MathWorks, USA) and further postprocessed in OriginPro 2021b (OriginLab Corp, USA). Statistical analysis was also performed using OriginPro software.Results and discussion
HyperCEST depletion spectra of CB6 in the PBS solutions are shown in Fig.1. The HyperCEST depletion occurred when the depolarization pulses were applied at -70ppm (known resonance frequency of HP 129Xe encapsulated in CB6). Utilization of the 16x30ms 330o 3LS pre-pulse train allowed for detection of all the concentrations down to 25uM (10% HyperCEST depletion). Only 3.4% depletion was observed from the 10uM sample and cannot be counted as a valid HyperCEST effect since this change was below the noise level. On the other hand, 16x30ms sinusoidal depolarization pulses allowed clear detection of the 10uM solution: 16% HyperCEST depletion was observed from the 10uM CB6 sample. Utilization of the sinusoidal RF pulses in the initial depolarization pulse train allowed reduction of the minimal detectable CB6 concentration at 3.0T by more than 3 times compared to that using the 3LS pulses.Changes in the HyperCEST effect with varying CB6 concentrations are shown in Fig.2. In addition to a comparison between the two different pulse trains used in this study, we also compared our results to results previously obtained by Hane et. al9. The strength of the HyperCEST effect from the 1mM solution after utilization of the sinusoidal pulses was approximately 100%, which is two times larger compared to that using the 3LS pulse train employed by Hane et. al2. Whereas the HyperCEST efficiency of the 3LS pulses decreased more than 3 times after a 10 times reduction in CB6 concentration, utilization of the sinusoidal pre-pulse train resulted in a 70% HyperCEST depletion. Utilization of the depolarization sinusoidal pulse train maximized the HyperCEST effect for all the CB6 concentrations. A paired t-test (Fig.3) revealed that the use of the 16x30ms sinusoidal pre-pulse train yields significantly better HyperCEST performance for the CB6 molecule (p<0.01). In addition, the 16x30ms 330O 3LS pulses used in this study demonstrated significantly better performance compared to 3LS pulses previously used in the literature2 (p=0.015).
Overall, the HyperCEST detection limit for conventional 3LS saturation pulses was found to be 0.025mM. Changing the saturation pre-pulse train to sinusoidally-shaped pulses allowed maximization of the HyperCEST effect for CB6 molecules resulting in a CB6 detection limit reduction below the 0.01mM. These results are of paramount importance since they demonstrate the potential for significant reduction of the CB6 injection dose for further in vivo experiments. This will facilitate further clinical translation of HyperCEST MRI for molecular imaging.
Acknowledgements
This study was funded by a Natural Science Engineering Research Council Discovery grant (RGPIN-2017-05359). Yurii Shepelytskyi was supported by a MITACS Elevate postdoctoral fellowship (IT25574). Vira Grynko was supported by an Ontario Trillium Scholarship. The authors acknowledge Xemed LLC for their continuous support and assistance with the polarizer.
References
1. Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science (80- ). 2006;314(5798):446-449. doi:10.1126/science.1131847
2. Hane FT, Li T, Smylie P, et al. In vivo detection of cucurbit[6]uril, a hyperpolarized xenon contrast agent for a xenon magnetic resonance imaging biosensor. Sci Rep. 2017;(3):41027. doi:10.1038/srep41027
3. Hane FTFT, Fernando A, Prete BRJBRJ, et al. Cyclodextrin-Based Pseudorotaxanes: Easily Conjugatable Scaffolds for Synthesizing Hyperpolarized Xenon-129 Magnetic Resonance Imaging Agents. ACS Omega. 2018;3(1):677-681. doi:10.1021/acsomega.7b01744
4. Fernando PUAI, Shepelytskyi Y, Cesana PT, et al. Decacationic pillar[5]arene: A new scaffold for the development of 129Xe MRI imaging agents. ACS Omega. 2020;5(43):27783-27788. doi:10.1021/acsomega.0c02565
5. Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. Thermodynamics of xenon binding to cryptophane in water and human plasma. J Am Chem Soc. 2007. doi:10.1021/ja072965p
6. Schnurr M, Joseph R, Naugolny-Keisar A, et al. High Exchange Rate Complexes of 129 Xe with Water-Soluble Pillar[5]arenes for Adjustable Magnetization Transfer MRI. ChemPhysChem. 2018;20(2):246-251. doi:10.1002/cphc.201800618
7. Du K, Zemerov SD, Hurtado Parra S, Kikkawa JM, Dmochowski IJ. Paramagnetic Organocobalt Capsule Revealing Xenon Host-Guest Chemistry. Inorg Chem. 2020;59(19):13831-13844. doi:10.1021/acs.inorgchem.9b03634
8. McHugh CT, Kelley M, Bryden NJ, Branca RT. In vivo hyperCEST imaging: Experimental considerations for a reliable contrast. Magn Reson Med. 2021;(August):1-10. doi:10.1002/mrm.29032
9. Hane FT, Smylie PS, Li T, et al. HyperCEST detection of cucurbit[6]uril in whole blood using an ultrashort saturation Pre-pulse train. Contrast Media Mol Imaging. 2016;11(4):285-290. doi:10.1002/cmmi.1690
Figures
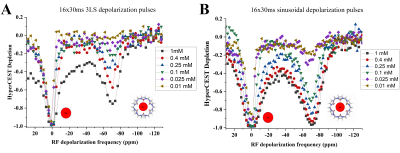
Figure 1. Depletion spectra of CB6 in PBS at different concentrations of the cage molecules. The spectra (A) were acquired using 16x30ms three-lobe sinc depolarization pulses of 330° FA. The spectra (B) were acquired utilizing 16x30ms sinusoidal RF pulses of 1530° FA. The HyperCEST effect from the CB6 molecules after irradiation with the sinusoidal pulse train was substantially stronger compared to conventional 3LS pulses.
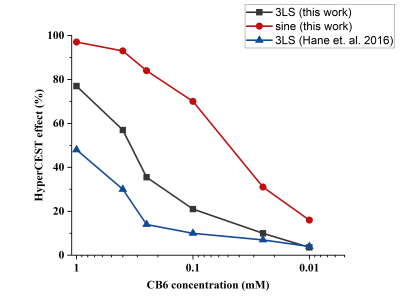
Figure 2. HyperCEST effect dependence on the concentration of CB6 in PBS. The lowest detectable concentration of CB6 using a conventional 3LS RF pulse train was 25uM. Utilization of sinusoidal saturation pulses allowed reduction of the lowest detectable concentration of CB6 to below 10 μM.

Figure 3. Statistical comparison of 16x30ms 3LS and sinusoidal RF saturation pre-pulse trains. The HyperCEST performance of sinusoidal RF pulses was significantly better compared to 3LS pulses (p < 0.01). 3LS pulses used in our study demonstrated significantly better performance compared to that previously used by Hane at. al (p = 0.015).