3568
Quantifying Ventilation Using Dynamic Xenon MRI During Free Breathing
Hooman Hamedani1, Faraz Amzajerdian1, Stephen Kadlecek1, Ryan Baron1, Kai Ruppert1, Ian Duncan1, Mostafa Ismail1, Yi Xin1, Tahmina Achekzai1, Luis Loza1, and Rahim Rizi1
1University of Pennsylvania, Philadelphia, PA, United States
1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
In order to image function in the free breathing lung with high temporal and special resolution, we have developed a passive, mechanically controlled MR-friendly gas delivery device capable of real time dosimetry and end-tidal gas measurements that can be used in combination with a non-rigid image registration technique. These dynamic images can thus be translated into comprehensible quantitative maps of lung ventilation and gas exchange. In order to image the lung continuously during normal breathing, we have also developed a fast-acquisition pulse sequence that uses diaphragm position to facilitate image reconstruction.
Introduction
Lung function imaging using polarized gases is traditionally performed during a long breath-hold following inhalation of a high concentration bolus of imaging gas in order to generate static images of gas distribution. However, imaging respiratory dynamics without a breath-hold and during normal breathing, using a small dose of hyperpolarized gas ad libitum, can provide a better understanding of the pathophysiology of various lung diseases, as it can dynamically depict the true physiological gas flow in patients without changing their breathing pattern. We developed a dynamic multibreath imaging regime that utilizes a lower dose of imaging gas—including developing the necessary tools and techniques to make this possible. This technique ameliorates the difficulties associated with breath-hold tolerance in diseased subjects with persistent cough and altered lung physiology, while also largely avoiding the anesthetic effects of large xenon doses. What is more, the advantages of such a protocol should be similar for functional lung imaging in pediatric populations. In this work, we present an approach for imaging lung ventilation with both high spatial and temporal resolution during a 5-minute free breathing protocol.Methods
Imaging was performed in a 1.5T Siemens scanner using an 8-channel 129Xe coil, with approval from Institutional Review Board. Images were acquired continuously during normal free breathing; subjects freely inhaled room air through a mouthpiece while 50 ml of the HP gas was added to each breath using a custom-made delivery system; 2.5 L total of HP gas was delivered during a complete 50-breath cycle. A 3D stack-of-spirals sampling pattern was used, which trades-off between temporal and spatial resolution with a satisfactory signal-to-noise [1]. Around 500 total images were acquired over 50 breaths in ~5 minutes (this varies between subjects depending on breathing rate). The TR/TE was set to 7.8/0.8ms, and a flip angle of 3˚ was applied to image 10 slices using an 80×80 matrix, achieving a resolution of 4.375×4.375×15 mm3. Diaphragm position over time was used to bin each individual image into 16 distinct phases for reconstruction during one breathing cycle. After registering all images to the end-inhale phase using a 3D nonrigid b-spline technique [2], the 16-phase periodic time-varying signal dynamics at each voxel were analyzed into harmonic amplitudes and phase components using Discrete Fourier Transforms (DFT) [3]. This analysis was used to produce average signal maps, as well as the phase map at which signal reaches its maximum.We account for signal dynamics during the breathing cycle using a model in which inhaled gas entering into each voxel contains the uniform magnetization density of the external gas source. Inhaled gas mixes with the residual gas, and this mixture is partially ejected on exhalation, resulting in gain or loss of magnetization proportional to the change in ventilated volume. It is convenient to describe signal and magnetization evolution in terms of normalized volumes at each step in the tidal breathing cycle: vj is the ventilated gas volume entering a region of tissue corresponding to an end-exhale voxel / end-exhale voxel volume, vfrc is end-exhale ventilated gas volume / end-exhale voxel volume, and Minf / Sinf refers to the magnetization or signal in a voxel completely filled with inhaled gas. In these terms, an individual voxel, coregistered to the lung at end-exhale, will contain increased magnetization going from time tj to tj+1 during inhalation as:
$$M_{j+1} = [M_j + M_{inf}(v_{j+1} - v_j)]e^{-\Gamma(t_{j+1} - t_j)}$$
and will lose signal during exhalation as:
$$M_{j+1} = M_j\frac{v_{j+1}}{v_j} e^{-\Gamma(t_{j+1} - t_j)}$$
The exponential factor in each expression accounts for O2/RF-induced spin-relaxation at combined rate $$$\Gamma$$$.
Signal is related to magnetization by a factor k (which may be spatially dependent based on local flip-angle or sensitivity) and an additional factor 1 / (1 - vfrc - vj) which accounts for the dilution and concentration of signal during expansion and contraction of tissue regions corresponding to end-exhale voxels. Note that the image registration does not account for this effect. By definition, vfrc is the minimum of the vj’s over the breathing cycle. Using this scheme, we can analyze the derived local volumes to yield regional tidal and residual volumes, as well as regional Fractional Ventilation (FV = vj / (vj + vfrc)).
Results and Discussion
3 healthy subjects were imaged using the above protocol. Figure 1 shows the gas delivery device, while Figure 2 shows the signal history during a 5-minute normal breathing session in which ~500 images were acquired, along with representative individual images and the diaphragm navigation curve. Figures 3A and 3B show the resulting 16 bin images for three representative slices pre- and post-registration, respectively. Figure 4 shows the signal in one cycle and the magnitude and phase of its DFT for the whole lung. Figure 5 shows quantified maps of regional ventilation from the top: a) average signal during the breathing cycle, b) regional tidal volume, c) regional residual volume, d) fractional ventilation and e) phase map. Interestingly, the lobar fissures can be detected due to the minute differences in lung function across adjacent lobes.Conclusion
Imaging during normal breathing is both more easily performed than static single-breath imaging during a non-physiologically long breath-hold, and better captures regional ventilation.Acknowledgements
No acknowledgement found.References
No reference found.Figures
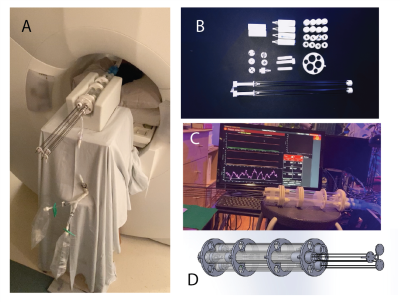
Figure 1. A) Gas delivery device during imaging with attached xenon gas. B) Internal components of the delivery device. C) Monitoring and calibration of the device. D) CAD drawing of the device.
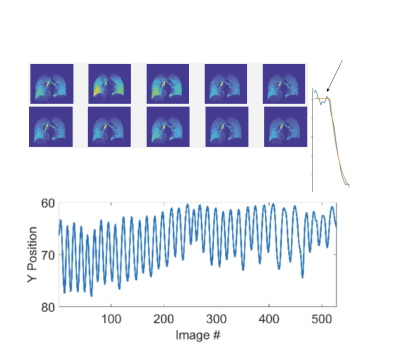
Figure 2. Diaphragm positions over time calculated from each individual image.
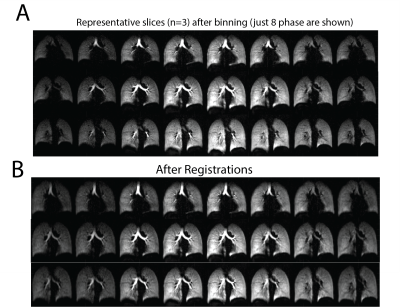
Figure 3. Three representative slices of the 16 binned images pre- and post-registration.
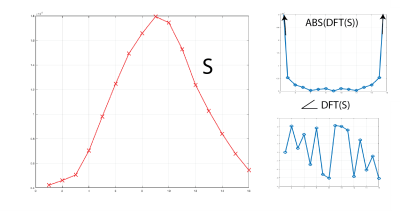
Figure 4. Signal of one breathing cycle along with the magnitude and phase of its DFT for the whole lung.

Figure 5. Quantified maps of regional
ventilation from the top: average signal during the breathing cycle, regional tidal volume, regional residual volume, fractional ventilation, and phase map.
DOI: https://doi.org/10.58530/2022/3568