3566
Order-Unity 13C Nuclear Polarization of [1-13C]Pyruvate in Seconds1Chemistry and Oncology, Wayne State University, Detroit, MI, United States, 2Chemistry, North Carolina State University, Raleigh, NC, United States, 3Chemistry, Wayne State University, Detroit, MI, United States, 4National Heart, Lung, and Blood Institute, Bethesda, MD, United States, 5National Cancer Institute, Bethesda, MD, United States, 6Southern Illinois University Carbondale, Carbondale, IL, United States
Synopsis
This presentation covers the recent advances in spin physics and instrumentation of Signal Amplification By Reversible Exchange (SABRE) in SHield Enables Alignment Transfer to Heteronuclei (SHEATH). Order unity 13C polarization for [1-13C]pyruvate was demonstrated for catalyst-bound species by SABRE-SHEATH, which becomes possible due to favorable 13C relaxation dynamics in a microtesla magnetic field. The magnetic field, temperature and co-solvents heavily modulate the attainable 13C polarization, providing an opportunity for optimization to deliver highly polarized [1-13C]pyruvate quickly and cheaply for biomedical applications. The design of clinical-scale hyperpolarizer is described for production of [1-13C]pyruvate and other metabolically relevant hyperpolarized contrast agents.
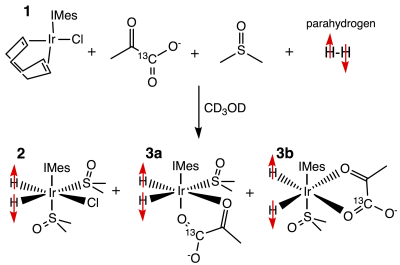
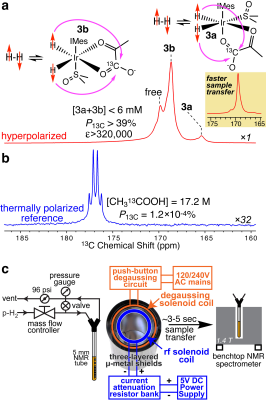
METHODS: [1-13C]pyruvate forms a complex with the standard catalyst used for SABRE, [IrCl(COD)(IMes)] (IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene; COD = cyclooctadiene)], Figure 1). The efficient hyperpolarization transfer from p-H2-derived hydrides to the 13C nuclear spins of [1-13C]pyruvate becomes possible by performing SABRE in sub-microtesla magnetic fields using SABRE in SHield Enables Alignment Transfer to Heteronuclei (SABRE-SHEATH).2,3 The overall diagram of the hyperpolarizer is provided in Figure 2c. The device (61x47x47 cm size) operates with either a static magnetic field using an internal DC power supply or a pulsed variable field using a commercially available wave-form generator.
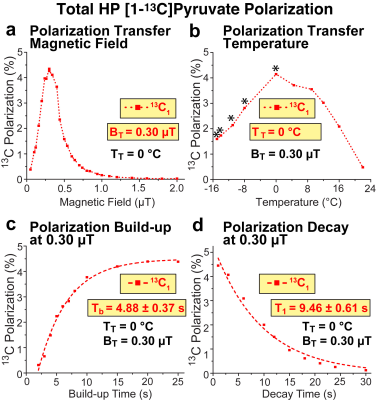
RESULTS AND DISCUSSION: Figure 2a shows a spectrum of a 6 mM Ir-[1-13C]pyruvate complex composed of both 3a and 3b with P13C of 39% at the time of detection; a corresponding 13C reference spectrum from thermally polarized neat [1-13C]acetic acid is shown in Figure 2b. Because the HP sample depolarizes during the 3-5-second long sample transfer (due to sample exposure in the Earth’s field and the 1.4 T field of the NMR spectrometer), the P13C value at the time of production (i.e., at the conclusion of the SABRE-SHEATH process) is estimated to be >50% on the catalyst-bound complexes 3a and 3b. Kinetically, the build-up to these high P13C levels becomes possible because the 13C T1 relaxation time of HP [1-13C]pyruvate in this complex at 0.30 microtesla is relatively long: 9.5 s (i.e., the spin relaxation rate is ~0.1 s-1) even at the relatively large catalyst concentration of 6 mM. As a result, the effective 13C polarization build-up constant, Tb = 4.9 s, corresponds to a build-up rate of ~0.2 s-1—substantially faster (Figure 3c) than the spin relaxation rate (Figure 3d), allowing one to achieve order-unity 13C polarization.
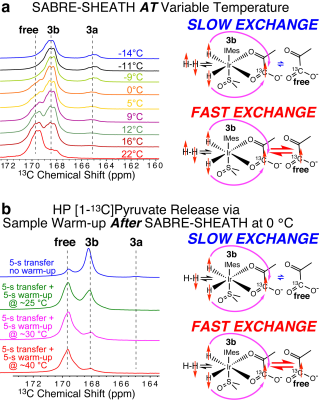
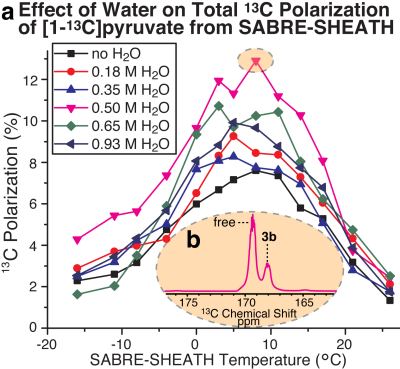
Optimization of the magnetic field and temperature of 13C SABRE-SHEATH for [1-13C]pyruvate (Figure 3) revealed the optimum polarization transfer field BT of ~ 0.3 microtesla and the optimum polarization transfer temperature TT of ~0 °C.Temperature has a profound effect on the exchange rates of [1-13C]pyruvate involving complex 3b, Figure 4a. Even though the 13C polarization was nearly fully locked in the bound 3b state at 0 °C (Figure 4b, blue trace), the 13C polarization bolus can be rapidly released if the HP solution’s container is rapidly warmed up after sample transfer from the shield, but before inserting the sample in the NMR detector. We also investigate the role of H2O as a potential secondary co-ligand or substrate modifier in SABRE-SHEATH experiments. Indeed, systematic H2O titrations revealed the dependence of P13C not only on DMSO concentration, but also on water concentration. Figure 5 shows a clear P13C modulation of [1-13C]pyruvate by varying the concentration of H2O added to the sample. The presence of H2O in an optimal concentration doubles P13C (compared to no water added), reaching ~13% for the free substrate at the optimum temperature.
CONCLUSION: Although future systematic optimization studies are certainly warranted to further improve 13C polarization efficiency of HP [1-13C]pyruvate produced via SABRE-SHEATH, this report clearly demonstrates that order-unity 13C polarization is fundamentally attainable. Moreover, the P13C value of ~13% for the free substrate is already useable for in vivo studies. Future in vivo feasibility studies will need to address remaining translational roadblocks by combining the present polarization techniques with ongoing efforts to achieve rapid SABRE catalyst removal and HP [1-13C]pyruvate reconstitution into biocompatible aqueous media of injectable solutions.
Acknowledgements
This work was supported by NSF CHE-1905341 and CHE-1904780, NCI 1R21CA220137, NIBIB R21EB025313 and R01EB029829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. T.T. acknowledges funding from the North Carolina Biotechnology Center and the Mallinckrodt Foundation.
References
1. Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science. 2009;323(5922):1708-1711.
2. Theis T, Truong ML, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J Am Chem Soc. 2015;137(4):1404-1407.
3. Barskiy DA, Shchepin RV, Tanner CPN, Colell JFP, Goodson BM, Theis T, Warren WS, Chekmenev EY. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem. 2017;18:1493–1498.
4. Iali W, Roy SS, Tickner BJ, Ahwal F, Kennerley AJ, Duckett SB. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew Chem Int Ed. 2019;58(30):10271-10275.
5. Gemeinhardt ME, Limbach MN, Gebhardt TR, Eriksson CW, Eriksson SL, Lindale JR, Goodson EA, Warren WS, Chekmenev EY, Goodson BM. “Direct” 13C Hyperpolarization of 13C-Acetate by MicroTesla NMR Signal Amplification by Reversible Exchange (SABRE). Angew Chem Int Ed. 2020;59(1):418-423.
6. Chapman B, Joalland B, Meersman C, Ettedgui J, Swenson RE, Krishna MC, Nikolaou P, Kovtunov KV, Salnikov OG, Koptyug IV, Gemeinhardt ME, Goodson BM, Shchepin RV, Chekmenev EY. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal Chem. 2021;93(24):8476–8483.
Figures