3565
Feasibility of Assessing Skeletal Muscle Metabolism of Muscular Dystrophy with Hyperpolarized 13C-Pyruvate1AIRC, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2Internal Medicine, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 3Radiology, UT Southwestern Medical Center at Dallas, Dallas, TX, United States
Synopsis
Pyruvate metabolism in skeletal muscle plays an essential role for energy production and development of muscular dystrophy (MD). However, investigation of the metabolism of MD has been focused on myocardium due to its vital importance, rather than skeletal muscle. This study presents preliminary results that demonstrate the feasibility of assessing altered pyruvate metabolism in the skeletal muscle of Becker MD patient with hyperpolarized [1-13C]pyruvate. In parallel, the metabolic profile of MD skeletal muscle was analyzed by NMR using a Duchenne MD mouse model after [U-13C3]pyruvate infusion. This study indicates that pyruvate metabolism is altered in skeletal muscle with MD.
INTRODUCTION
Muscular dystrophy (MD) is a group of diseases that cause progressive muscle weakness and atrophy. It is still unclear as to what extent energy metabolism affects muscle pathology of MD. In the skeletal muscle, the conversion of pyruvate to lactate, alanine and acetyl-CoA via lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and pyruvate dehydrogenase (PDH), respectively, is sensitive to its energy state. A better understanding of the metabolic balance and the accompanied alterations in the dystrophic muscle may improve establishing therapeutic strategies for MD patients. 13C MR with hyperpolarized (HP) [1-13C]pyruvate allows in vivo imaging of metabolic pathways of interest by detecting the relevant products such as lactate, alanine and bicarbonate1, 2. Feasibility of this technique for investigating skeletal muscle metabolism was studied by several groups2-6 and recently demonstrated in human calf muscle7. In particular, PDH and LDH activities were instantly responsive to exercise and pharmacological interventions (e.g., dichloroacetate)4, 5, 7. In this study, we explored the feasibility of HP pyruvate for assessing skeletal muscle metabolism of MD.MATERIALS and METHODS
The protocol of the human study was approved by the local Institutional Review Board (STU-2021-0168, STU-2018–0227). Informed written consents were obtained from a Becker MD patient (21 y.o., male, BMI = 22.3) and a healthy control subject with similar age and BMI (28 y.o., male, BMI = 21.6). The participants rested for one hour and moved to a 3T MRI scanner (750w Discovery, GE Healthcare) via wheelchair. The participants were positioned supine with one leg in a 13C/1H dual-frequency RF coil (Clinical MR Solutions, LLC) and imaged using a 13C/1H integrative MR protocol, which consists of series of 1H MRI and a 13C MRS with an injection of HP [1-13C]pyruvate at rest. Sample preparation, polarization, dissolution, and quality assurance were performed using a SPINlabTM polarizer (GE Healthcare) as previously described7. For 13C MRS, time-resolved free-induction-decay data were acquired using a slice-selective pulse-and-acquire sequence (slab thickness = 10 cm, TR = 3 s, spectral width = 10,000 Hz, #spectral point = 2,048, scan time = 4 min)7.In parallel, Duchenne MD mice (n = 3)8 and age-matched controls (n = 2) were prepared for an ex vivo NMR metabolomics study. A bolus of 0.36 mg/g sodium [U-13C3]pyruvate in 0.2-mL solution was given intravenously, followed by a two-hour infusion of 0.0083 mg/g sodium [U-13C3]pyruvate at 0.15 mL/hr. The infused mice were euthanized and the skeletal muscle tissue was harvested, then immediately freeze-clamped with liquid N2. 13C spectra of the tissue extracts were acquired using a 600MHz Oxford NMR system with a cryogenically-cooled 13C probe.RESULTS and DISCUSSION
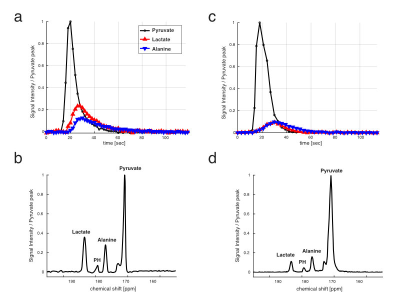
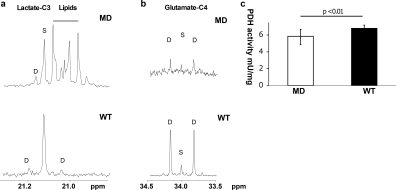
Time-averaged 13C spectra over the first 90 s from the study participants are shown in Fig 1. Lactate production was higher in the MD patient (Fig 1a, b) than the control subject (Fig 1c, d). The area under the HP [1-13C]lactate curve relative to the total HP 13C (TC) signal (sum of pyruvate, lactate, alanine, and bicarbonate) in the MD patient (lactate/TC = 0.28) was also larger than any of the healthy participants in our previous studies with healthy subjects (0.18 ± 0.06, n = 3). Conversely, [1-13C]pyruvate/TC was smaller in the patient (pyruvate/TC = 0.56) than the healthy subject (0.72) as well as the literature (0.66 ±0.05). The bicarbonate production was minimal, which is consistent with previous observation in resting muscle7.The ex vivo tissue NMR analysis showed larger 13C-labeing in lactate in MD mice (enrichment = 0.2 ± 0.04%) compared to wild-type (WT; 0.13 + 0.03%), as shown in C3 lactate doublet production (Fig 2a), which was consistent with the upregulated in vivo LDH activity seen in MD patient. In addition, we could detect pyruvate flux via PDH from C4 glutamate labeling. The C45 doublet was lower in MD (3.7 ± 0.4%) compared to WT (10.4 ± 1.5%) (Fig 2b), suggesting decreased PDH activity in MD muscle. PDH enzyme activity assay was also performed for the mouse skeletal muscle tissue, and MD mouse models showed significantly less enzyme activity (p <0.01, Fig 2c). The lipid peaks near C3 lactate were specific to MD mice and did not appear in the skeletal muscle of WT. This is because the muscle fiber in the skeletal muscle is gradually replaced by fat as MD progresses9. Increased lactate production and upregulated LDH are associated with inflammation in many diseases10. Although the metabolic contribution to inflammation is unclear, inflammation is a central feature in MD11. Mitochondria are also known to undergo significant metabolic changes in MD, including decreased oxidative phosphorylation12. Thus, metabolic study of MD model may lead to discovery of new metabolic biomarkers as noninvasive diagnostic tools to monitor disease progression and treatment response.CONCLUSION
This study reports our preliminary observations of pyruvate metabolism in skeletal muscle of MD. The animal and patient studies collectively suggest that pyruvate metabolism is favorable to lactate formation rather than pyruvate oxidation. As the patient study was performed at rest, essentially no bicarbonate production was detected. The future study will focus on HP studies with MD patients at rest and during mild plantar flexion exercise.Acknowledgements
The Paul D. Wellstone Muscular Dystrophy Cooperative Research Center; National Institutes of Health of the United States (R01 NS107409, P41 EB015908, S10 RR029119, S10 OD018468); The Welch Foundation (I-2009-20190330).References
1. Gallagher, F.A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940-943 (2008).
2. Golman, K., in 't Zandt, R. & Thaning, M. Real-time metabolic imaging. Proc Natl Acad Sci U S A 103, 11270-11275 (2006).
3. Bengtsen, M.B. et al. Hyperpolarized [1-(13) C]pyruvate combined with the hyperinsulinemic euglycemic and hypoglycemic clamp technique in skeletal muscle in a large animal model. Exp Physiol (2021).
4. Park, J.M. et al. Hyperpolarized NMR study of the impact of pyruvate dehydrogenase kinase inhibition on the pyruvate dehydrogenase and TCA flux in type 2 diabetic rat muscle. Pflugers Arch (2021).
5. Park, J.M. et al. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol 218, 3308-3318 (2015).
6. Leftin, A., Roussel, T. & Frydman, L. Hyperpolarized functional magnetic resonance of murine skeletal muscle enabled by multiple tracer-paradigm synchronizations. PLoS One 9, e96399 (2014).
7. Park, J.M. et al. Hyperpolarized (13)C MR Spectroscopy Depicts in Vivo Effect of Exercise on Pyruvate Metabolism in Human Skeletal Muscle. Radiology, 204500 (2021).
8. Grady, R.M. et al. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, 729-738 (1997).
9. Li, W. et al. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul Disord 25, 375-380 (2015).
10. Drent, M., Cobben, N.A., Henderson, R.F., Wouters, E.F. & van Dieijen-Visser, M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 9, 1736-1742 (1996).
11. Serra, F. et al. Inflammation in muscular dystrophy and the beneficial effects of non-steroidal anti-inflammatory drugs. Muscle Nerve 46, 773-784 (2012).
12. Hughes, M.C. et al. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H(2) O(2) emission during impaired oxidative phosphorylation. J Cachexia Sarcopenia Muscle 10, 643-661 (2019).
Figures