3564
Hyperpolarized [1-13C]Pyruvate MRI Using a Metabolite-Specific Rotated Echo-Planar Imaging Sequence with Half Scan1University of California San Francisco, San Francisco, CA, United States, 2HeartVista, Los Altos, CA, United States
Synopsis
Hyperpolarized 13C-labeled compound is an emerging technique in biomedical imaging to non-invasively detect metabolic processes and has been translated in clinical for cancer and cardiac metabolism studies. However, hyperpolarized MRI signal is limited by high spatial and temporal resolution and high SNR. This work describes a novel rotated Echo-planar imaging (EPI) trajectory with a half scan acquisition that shortens echo time to reduce the T2* effect in metabolic imaging. Compared to the standard full sampled EPI trajectory, the proposed trajectory has the advantage of gaining higher signal intensity, and is less sensitive to motion artifacts, distortion artifacts and aliasing artifacts.
Introduction
MRI of hyperpolarized 13C-labeled compounds is an emerging technique in biomedical imaging to non-invasively detect metabolic processes.[1,2] Metabolite-specific imaging using spectral-spatial excitation of a single resonance with an echo-planar imaging (EPI) readout is one common trajectory for hyperpolarized imaging that provides rapid, volumetric coverage of multiple metabolites with efficient sampling. However, EPI readouts are sensitive to B0 inhomogeneity and suffer from the T2* decay effect, which limits the SNR of 13C imaging.[3] In this study, we proposed a rotated EPI trajectory with half scan to shorten echo time and reduce T2* effect. At the same time, rotated trajectory is less sensitive to the motion, distortion, and Nyquist ghost artifacts.[4,5]Methods
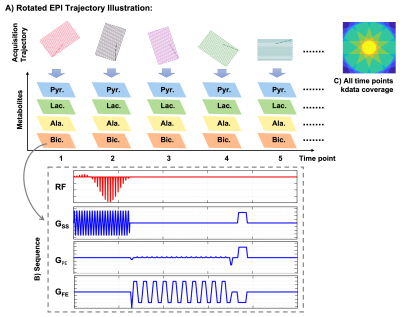
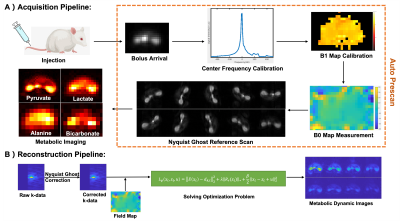
Acquisition Design: The proposed metabolite-specific sequence(Fig.1) consists of a spectral-spatial RF pulse[6], EPI readout, and spoiler gradients. The EPI readout with partial-Fourier coverage samples data in a rotating pattern in the dynamic domain.[4] Considering the C13 data have higher SNR only in the first approximately 30s, we used 10 timepoints with rotation as the rotation period.The acquisition(Fig.2A) started immediately after injection. After detecting bolus arrival, and calibrating the pyruvate center frequency and B1[6], we used a multiple-echo sequence to measure B0 map with pyruvate signal. Considering the gradient delay varies during the rotation, one slice of reference data was acquired for Nyquist ghost correction. After the above autonomous pre-scan, the proposed metabolite-specific sequence was triggered automatically to acquire metabolic imaging data.
Reconstruction Design: The reconstruction process starts from Nyquist ghost correction[7]. Considering all timepoints signal were derived from the Fourier Transform of the same subject, we relocated all k-data by[8,9]$$r\left(k_m^\prime,k_n^\prime\right)=r(k_m,k_n)e^{i\frac{\pi}{5}t}\qquad(1)$$for the tth time point. With the discrete Fourier Transform, all timepoints data can be designed into an encoding matrix and reformed into an Augmented Lagrangian equation:$$L_p\left(x_t,z_t,u\right)=\parallel{E(x_t)-d_{k,t}}\parallel_2^2+{\lambda}\parallel{R_r(z_t)}\parallel_*+\frac{\beta}{2}\parallel{x_t-z_t+u}\parallel_2^2\qquad(2)$$where $$$R_r{(z}_t) $$$ is a locally low-rank regularization[10], $$${\lambda},{\beta}$$$ are penalty parameters, $$$u$$$ is dual variable, $$$E(x_t)$$$ is joint combined encoding operator[8,9]$$E\left(x_t\right)= \sum_m\sum_n x_t\left(m,n\right)e^{-j({mk}_m^\prime+nk_n^\prime)}e^{i∆BT_n}\qquad(3)$$where $$$∆B$$$ is field map, $$$T_n$$$ is the readout time at nth phase encoding k-line. Equation(2) can be rewritten into optimization problems and solved by ADMM[11].
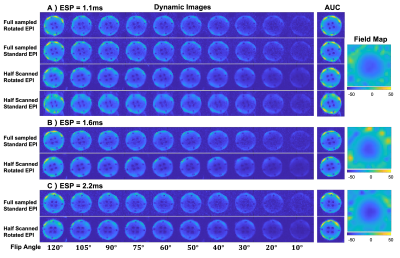
Phantom Study: 13C ethylene glycol phantom data were acquired on a GE 3T scanner(Waukesha, WI) with QTAR coil(Clinical MR Solutions) using a commercial software platform for research sequence development(RTHawk, HeartVista, CA). To simulate data inconsistency of hyperpolarized carbon signal in dynamic direction, we used variable flip angles(Fig.3) to excite carbon signal with ten timepoints. Four different EPI trajectories were compared: full sampled standard EPI, full sampled rotated EPI, half scanned standard EPI, half scanned rotated EPI. Other parameters: FOV=32×32cm2, ESP=1.1/1.6/2.2ms, BW=50/25/25kHz, NEX=50, TR=500ms, timepoint=10. Field maps were acquired by a multiple-echo GRE sequence.
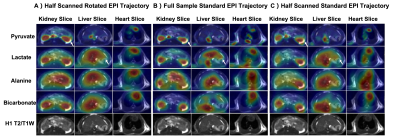
Animal Study: Animal data were acquired with the same system except for using a single channel rat coil. The study was performed on a healthy Sprague-Dawley rat with three identical injections of hyperpolarized [1-13C]pyruvate(SpinLab, GE healthcare) to compare pyruvate/lactate/alanine/bicarbonate signals acquired by three different EPI trajectories(full sampled standard EPI, half scanned standard EPI, half scanned rotated EPI). Hyperpolarized data were acquired with variable resolutions[12], pyruvate signal was acquired with 2.5×2.5mm2 in-plane resolution (FOV=8×8cm2), lactate at 5.0×5.0mm2, and alanine/bicarbonate at 7.5×7.5mm2. Other parameters are: matrix size=32×20(half scan)/32×32(full sample), TE=17.44(half scan)/43.84(full sample)ms, ESP=2.2ms, TR=110ms, slice=6, temporal resolution=2.64s, totally 30 timepoints. Proton data were acquired by a FIESTA sequence.
Results
Fig.3 shows phantom results with three different ESPs. With the ESP increasing, distortion artifacts become more severe and are challenging to remove. However, with the rotated EPI trajectory, distortion artifacts correction has a better performance than the standard EPI trajectory. Fig.3A) shows a comparison of results with four trajectories. With the half scan acquisitions, data suffers less T2* signal losses. However, high spatial frequency errors were still visible after partial-Fourier reconstruction when using half scanned standard EPI.Fig.4 shows the animal results with three different EPI trajectories. The carbon area under the curve(AUC) images of half scan rotated EPI show a better overlay performance on the proton images than the other two trajectories(white arrows). Heart slice carbon AUC images show that rotated EPI is less sensitive to heartbeat motion. Bicarbonate kidney and liver slice AUC images of rotated EPI show an overall signal improvement compared to full sampled standard EPI due to the shortened TE. Left kidney AUC images of half scanned standard EPI show a high-frequency estimation error after partial-Fourier reconstruction.
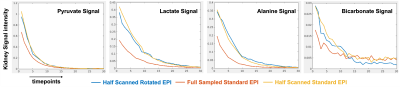
Fig.5 shows dynamic curves of pyruvate/lactate/alanine/bicarbonate signal. With half scan acquisition, all hyperpolarized carbon signals suffer less T2* decay than the full sample acquisition. Based on the last 10 timepoints of the bicarbonate signal curve, the locally low-rank regularization shows a noise suppression effect.
Discussion & Conclusion
This study employed a rotated EPI trajectory with half scan for a shorter echo time than full sampled standard EPI with the same resolution, which can reduce the T2* effect of hyperpolarized carbon signal and improve the signal intensity. Meanwhile, the rotated trajectory can provide motion and distortion insensitivity. In future studies, we will estimate phase errors of Nyquist ghost with parallel imaging methods and add the phase errors into the encoding matrix for a better reconstruction performance. To summarize, rotated EPI with half scan shows a better image quality than full sampled standard EPI with the same resolution.Acknowledgements
This work was supported by NIH Grants P41EB013598, R21DK130002, R01CA249909, U01EB026412, and American Cancer Society Grant RSG‐18‐005‐01‐CCE.References
[1] Ardenkjær-Larsen, Jan H., et al. "Increase of signal-to-noise of more than 10,000 times in liquid state NMR." Discovery medicine 3.19 (2003): 37-39.
[2] Golman, Klaes, and Mikkel Thaning. "Real-time metabolic imaging." Proceedings of the National Academy of Sciences 103.30 (2006): 11270-11275.
[3] Yen, Yi‐Fen, et al. "T2 relaxation times of 13C metabolites in a rat hepatocellular carcinoma model measured in vivo using 13C‐MRS of hyperpolarized [1‐13C] pyruvate." NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 23.4 (2010): 414-423.
[4] Pipe, James G. "Motion correction with PROPELLER MRI: application to head motion and free‐breathing cardiac imaging." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 42.5 (1999): 963-969.
[5] Wang, Fu‐Nien, et al. "PROPELLER EPI: an MRI technique suitable for diffusion tensor imaging at high field strength with reduced geometric distortions." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 54.5 (2005): 1232-1240.
[6] Tang, Shuyu, et al. "A regional bolus tracking and real‐time B1 calibration method for hyperpolarized 13C MRI." Magnetic resonance in medicine 81.2 (2019): 839-851.
[7] Chen, Nan‐Kuei, Alexandru V. Avram, and Allen W. Song. "Two‐dimensional phase cycled reconstruction for inherent correction of echo‐planar imaging Nyquist artifacts." Magnetic resonance in medicine 66.4 (2011): 1057-1066.
[8] Liu, Xiaoxi. "Advanced High Resolution Diffusion Tensor Magnetic Resonance Imaging". Diss. The University of Hong Kong, 2020.
[9] Liu, X., et al. "Self-calibrated and Collaborative Propeller-EPI Reconstruction (SCOPER) for High-Quality Diffusion-Tensor Imaging." International Society for Magnetic Resonance in Medicine (ISMRM 27th Annual Meeting & Exhibition, 2019. International Society for Magnetic Resonance in Medicine., 2019.
[10] Tamir, Jonathan I., et al. "Targeted rapid knee MRI exam using T2 shuffling." Journal of Magnetic Resonance Imaging 49.7 (2019): e195-e204.
[11] Boyd, Stephen, Neal Parikh, and Eric Chu. Distributed optimization and statistical learning via the alternating direction method of multipliers. Now Publishers Inc, 2011.
[12] Gordon, Jeremy W., et al. "A variable resolution approach for improved acquisition of hyperpolarized 13C metabolic MRI." Magnetic resonance in medicine 84.6 (2020): 2943-2952.
Figures