3555
Quantitative Susceptibility Mapping Artifact Reduction in Ex Vivo Tissue Specimens Using Adaptive Multi-Contrast Informed Regularization1Electrical and Computer Engineering, CORNELL UNIVERSITY, New York City, NY, United States, 2Department of Radiology, Weill Cornell Medicine, New York City, NY, United States, 3Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, Jinan, Shandong, China, 4Department of Radiology, Hainan General Hospital, Hainan Hospital Affiliated to Hainan Medical College, Hainan, China, 5Department of Pathology and Laboratory Medicine, Weill Cornell Medicine and New York Presbyterian Hospital, New York City, NY, United States
Synopsis
Quantitative susceptibility mapping (QSM) solves an ill-posed dipole field inversion problem and is prone to streaking and shadowing artifacts. This report presents a novel QSM reconstruction algorithm that effectively suppresses these artifacts using prior information from traditional MRI contrasts. The proposed algorithm was evaluated using ex vivo high resolution QSM of cerebellum and brainstem specimens using COSMOS as the reference method, showing improved QSM accuracy and artifact reduction and better agreement with myelin sensitive Luxol Fast Blue staining compared to conventional MEDI and MEDI-SMV methods.
Summary of Main Findings
A new QSM algorithm, ampQSM is evaluated that incorporates multi-contrast inputs to reconstruct susceptibility. This algorithm is tested on ex vivo human brain tissue specimen datasets of the cerebellum and brainstem at high resolution sub-millimeter 3T MRI. COSMOS ground truth models and Luxol Fast Blue staining of tissue specimens in addition to clinical scores suggest that ampQSM performs superiorly compared to competing techniques by reducing error and artifact incidence.Introduction
Quantitative susceptibility mapping (QSM) is an MRI method to compute the magnetic susceptibility map of tissue from the tissue magnetic field derived from the complex gradient echo image data. However, for ex vivo data, QSM quality can suffer in the presence of air and other sources of noise and desired reconstruction quality should produce sub-millimeter high resolution spatial information without strong artifacts. The objective of this study was to develop an accurate and robust reconstruction algorithm for ex vivo brain QSM which utilizes images acquired with traditional MRI contrasts, such as T1-weighted (T1w) and proton density-weighted (PDw) images, as Bayesian priors to suppress streaking and shadowing artifacts. The proposed algorithm known as adaptive multi-parameter QSM, or ampQSM was evaluated by comparing with several existing algorithms that utilize local field dipole inversion, including MEDI-PDF (termed MEDI)1,2, and MEDI-PDF-SMV (termed MEDI-SMV)1,2.Methods
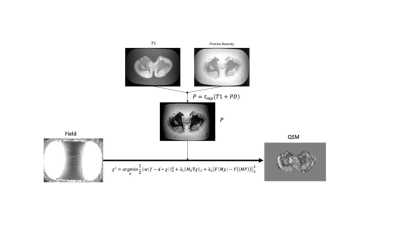
The proposed ampQSM algorithm is an extension of the MEDI local field inversion algorithm and computes the susceptibility map from the local field by iteratively solving the following minimization problem.$$\chi^* = argmin_\chi \frac{1}{2} ||w(f-d * \chi)||^2_2 + \lambda_2 ||M_G \nabla \chi ||_1 + \lambda_2 ||F(M \chi) - F(MP)||^2_2 \ \ \ \ [1] $$
In the last term in Eq.1, M is the binary tissue mask, F is a filtration function, and P is a composite image derived from T1w and PDw images as follows,
$$P = t_{rw}(T1w_{rw} + PDw_{rw}) \ \ \ \ [2] $$
where $$$T1w_{rw}$$$ and $$$PDw_{rw}$$$ reweights the intensity value of T1w and PDw images by rescaling each to range from 0 to 1, then the function $$$t_{rw}$$$ is applied, which takes $$$mean(T1w_{rw} + PDw_{rw}) = a$$$ and $$$0.75max(T1w_{rw} + PDw_{rw}) = b$$$ and rescales as $$$P = \frac{0.2}{b}(T1w_{rw} + PDw_{rw} + \frac{0.1}{a})$$$. This allows the T1w and PDw to have equal weighting and the resultant map to be approximately the range of healthy tissue susceptibility. The operator is a filtration function, inspired by TFIR framework3, that is defined as
$$F = SMV(5mm) \ \ \ \ [3]$$
Figure 1 shows the schematic of the proposed ampQSM algorithm.
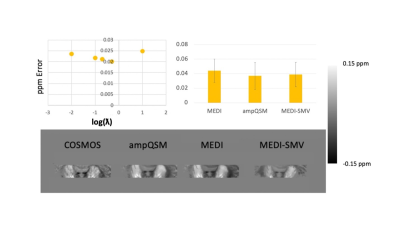
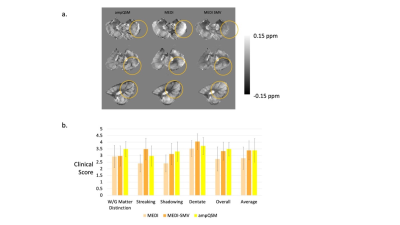
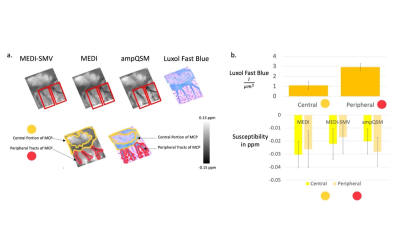
T1w 3D TSE sequence was utilized with a phase acceleration factor of 2, 4 excitations, resolution of 0.5x0.5x0.6 mm, 16/541 ms TE/TR. PDw 2D FSE sequence was utilized with 4 excitations, resolution of 0.5x0.5x0.6 mm, 14/4000 ms TE/TR. The multi-echo GRE was obtained at 0.5x0.5x0.6 mm resolution, with the first echo at 3.9 ms, phase acceleration of 2, and a total of 8 echoes with 4.7 ms echo spacing. Note that the T1w contrast resembles inversion recovery, white matter nulled sequences. This is due to the fixation process with formalin changing the recovery times of white and gray matter. COSMOS ground truth comparisons are performed utilizing a smaller specimen of a formalin fixed brain that fits into a 50 mL centrifuge tube in order to easily perform multiple orientation positioning.4 As such only the Dentate Nucleus region is displayed in the ground truth analysis figure. For comparison algorithms and ampQSM, the parameters used are MEDI PDF with $$$\lambda_1 = 0.001$$$, MEDI SMV with $$$\lambda_1 = 0.001$$$, SMV = 5mm kernel, ampQSM with $$$\lambda_1 = 0.001$$$ and $$$\lambda_2 = 0.0002$$$ , optimized against the ground truth root mean square error (RMSE). 7 cerebellums and 2 brainstems were reconstructed using MEDI-SMV, MEDI, and ampQSM. Parameters are identical to the COSMOS analysis. Clinical Scoring is performed, with three clinicians scoring each reconstruction for all 9 specimens in 5 different categories. Luxol Fast Blue staining is performed for all specimens of the cerebellum. The staining intensity of the central and peripheral tract areas is compared with each of the QSM reconstruction susceptibilities for the middle cerebellar peduncle through region of interest ROI segmentation. 5,6
Results and Discussion
COSMOS ground truth as depicted in Figure 2 shows that the error is reduced for ampQSM reconstructions compared to competing methods. Figure 3 depicts the comparison across methods, circled regions indicate differences in artifact incidence and clinical scores. ampQSM matches and exceeds the quality of MEDI and MEDI-SMV in various categories without the need to utilize the 5mm erosion kernel. Figure 4 depicts all three reconstructions along with the Luxol Fast Blue Staining Intensity, with ampQSM revealing better depiction and continuity of peripheral myelinated tracts as defined in the red outlined regions. The ground truth stained sections show that peripheral tracts have higher myelination density, and only ampQSM agrees with this result, while MEDI and MEDI-SMV fail to reveal this trend.Conclusion
ampQSM presents an improvement in error and artifact incidence for ex vivo brain specimen reconstructions through tests with ground truth models and clinical scores. Quantifications of Luxol Fast Blue stained sections further validates the potential of ampQSM in producing accurate and low artifact QSM reconstructions of brain specimens.Acknowledgements
The authors acknowledge all funding agencies and the Department of Radiology Weill Cornell, Department of Pathology and Laboratory Medicine Weill Cornell, and the CBIC City Group Biomedical Imaging Center.References
1. Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2015;73(1):82–101.
2. Kee Y, Liu Z, Zhou L, Dimov A, Cho J, De Rochefort L, Seo JK, Wang Y. Quantitative susceptibility mapping (QSM) algorithms: mathematical rationale and computational implementations. IEEE Transactions on Biomedical Engineering. 2017 Sep 5;64(11):2531-45.
3. Balasubramanian PS, Spincemaille P, Guo L, Huang W, Kovanlikaya I, Wang Y. Spatially Adaptive Regularization in Total Field Inversion for Quantitative Susceptibility Mapping. iScience [Internet]. 2020;23(10):101553. Available from: https://doi.org/10.1016/j.isci.2020.101553
4. Liu T, Spincemaille P, De Rochefort L, Kressler B, Wang Y. Calculation of susceptibility through multiple orientation sampling (COSMOS): A method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med. 2009;61(1):196–204.
5. Sjöbeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in azheimer’s disease - A neuropathological study. Int J Geriatr Psychiatry. 2005;20(10):919–26.
6. Warntjes JBM, Persson A, Berge J, Zech W. Myelin detection using rapid quantitative MR imaging correlated to macroscopically registered luxol fast blue-stained brain specimens. Am J Neuroradiol. 2017;38(6):1096–102.
Figures