3548
NOVEL POINT-OF-CARE MAGNETIC RESONANCE TECHNOLOGY TO MEASURE LIVER FAT: PHANTOM AND FIRST-IN-HUMAN PILOT STUDY1Radiology, UCSD, San Diego, CA, United States, 2Internal Medicine, UCSD, San Diego, CA, United States, 3Livivos Inc, San Diego, CA, United States
Synopsis
LiverScope® is a novel point-of-care (POC) NMR technology for liver fat quantification.
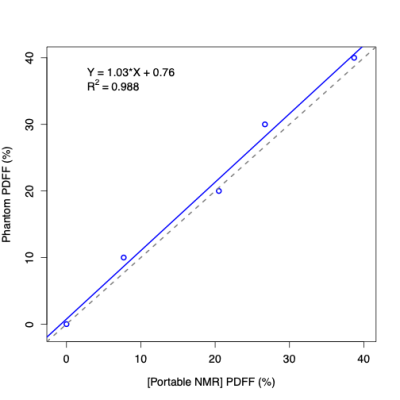
· POC NMR PDFF agreed closely with ground-truth PDFF values in commercial PDFF phantoms (R2 = 0.99)
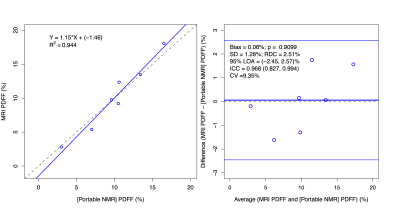
· POC NMR was feasible in an outpatient clinic, had no demonstrated adverse events, and agreed closely with contemporaneous MRI-PDFF reference standard values (R2 = 0.94) in human adults with obesity and at risk for NAFLD:
Introduction
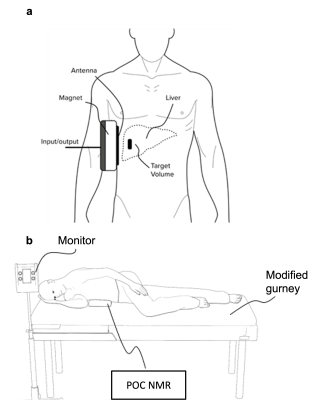
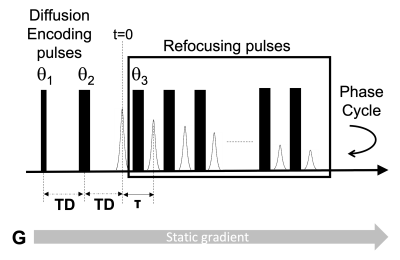
Nonalcoholic fatty liver disease (NAFLD) is increasing in prevalence (1–3). The current noninvasive gold standard for steatosis assessment is chemical shift encoded (CSE) magnetic resonance imaging-based proton density fat fraction (MRI-PDFF). A well-tolerated point-of-care (POC) test that is accurate and reliable could enhance clinical diagnosis, facilitate clinical trial screening, and expand access to advanced diagnostic technology. Liverscope® is a novel MR-based technology developed to measure PDFF, with POC capability (US20180220949 and US20190076080) (POC NMR). It uses a custom-shaped open permanent magnet array (0.2 Tesla) integrated with a standard clinical gurney. To measure PDFF, fat-water contrast is generated by measuring diffusivity of fat and water in the liver using a range of b-values. Clinical MRI systems cannot separate water and fat signals based on diffusivity differences because they cannot achieve this range of b-values. By comparison, POC NMR can generate fat-water diffusion contrast because it applies a strong constant field gradient able to produce sufficiently high b values (over 15,000 s/mm2). To detect signals from fat and water, POC NMR applies a modified CPMG pulse sequence. The signals can then be fitted to determine the fraction of fat in the measured area. This study investigates the feasibility and accuracy of POC NMR-PDFF in phantoms and in a first-in-human pilot study. For the human study, adults with obesity were recruited, POC NMR in a point-of-care setting was performed, and PDFF was measured contemporaneously with magnitude-data confounder-corrected chemical-shift-encoded MRI (MRI-M) - as reference.Methods
POC NMR-PDFF measurements were made on certified phantoms with known PDFF values (0 to 40%). In an IRB-approved, HIPAA-compliant prospective pilot study, we enrolled a convenience sample of adult patients from a comprehensive obesity treatment center between 11/2020 and 06/2021. Inclusion criteria were body mass index (BMI) >= 30 kg/m2 and willingness to undergo contemporaneous POC NMR and MRI PDFF. Liver PDFF was measured by POC NMR and, within 35 days after, by a confounder-corrected chemical-shift-encoded MRI PDFF acquisition and reconstruction method. Adverse events were documented. Linear regression analyses and Bland-Altman analyses were performed.Results
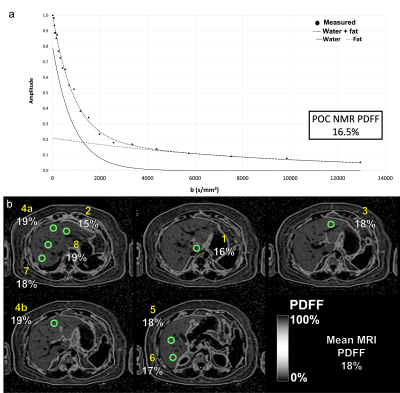
POC NMR-PDFF measurements agreed with known phantom PDFF values (R2=0.99). Fourteen participants enrolled in the pilot study. One withdrew before any procedures. MRI-PDFF could not be obtained in 4 participants (intra-exam claustrophobia reactions, n=3; exceeded MR scanner bore diameter, n=1). POC NMR was unevaluable in 2 participants (insufficient signal penetration depth, n=1; motion, n=1). In the remaining 7 participants (4 female; aged 31-74 years; median BMI 35 kg/m2), MRI-PDFF values ranged from 2.8 to 18.1%, POC NMR-PDFF values ranged from 3 to 25.2%, and POC NMR-PDFF measurements agreed with MRI-PDFF values (R2=0.94). POC NMR had no adverse events.Discussion
POC NMR performed in a point-of-care setting was feasible and agreed closely with reference values in phantoms and in individuals with obesity. This pilot study is limited in the size and PDFF range in the human study cohort. Only 7 participants had evaluable POC NMR PDFF and MRI PDFF measurements.Conclusion
POC NMR measures PDFF accurately in phantoms and, in a first-in-human pilot study, is feasible and accurate in adults with obesity. Further testing to determine precision and accuracy across larger and more diverse cohorts is needed.Acknowledgements
MB: 5T32EB005970-14
References
1. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. Elsevier Inc.; 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038.
2. Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x.
3. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: The third national health and nutrition examination survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. doi: 10.1093/aje/kws448.
4. Ninla Elmawati Falabiba. Single-Sided NMR. Casanova F, Perlo J, Blümich B, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. doi: 10.1007/978-3-642-16307-4.
Figures