3541
Accurate thermometry using hyperpolarized 129Xe free induction decay and spin-echo chemical shift imaging in rats1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany, 3Institute for Laboratory Animal Science and Central Animal Facility, Hannover Medical School, Hannover, Germany, 4Department of Clinical Airway Research, Fraunhofer Institute for Toxicology and Experimental Medicine, Hannover, Germany, 5Department of Respiratory Medicine, Hannover Medical School, Hannover, Germany
Synopsis
Thermometry using the proton resonance frequency (PRF) is challenging in inhomogeneous, fatty tissues and moving organs. Purpose of this study was to develop and test a combined free induction decay (FID)/spin-echo chemical shift imaging sequence for accurate hyperpolarized 129Xe MR thermometry in rats exposed to hypothermia. Significant changes of 129Xe lipid chemical shift and good agreement with core body temperature are observed. Bland–Altman analysis shows narrower limits of agreement and reduced mean difference for spin-echo acquisitions, suggesting improved precision and accuracy in fatty tissues. The method may be valuable as reference standard for development of more accurate 1H MR thermometry.
Introduction
Cancer is among the leading causes of death worldwide.1 Image-guided thermal ablation enables noninvasive treatment of unresectable tumors but requires accurate knowledge of temperature to ensure destruction of tumor tissue while preventing damage in surrounding healthy tissue.2Thermometry using 1H MRI measurements of proton resonance frequency (PRF) is a tool for non-invasive temperature measurements during thermal ablation.3 However, PRF thermometry remains challenging in inhomogeneous, fatty tissues, due to changes in magnetic susceptibility.4 Additionally, histology is needed to verify precision of PRF thermometry in ablation areas.5
The resonant frequency of 129Xe is highly sensitive to temperature and especially lipid-dissolved 129Xe exhibits a very strong temperature shift.6 More recently, Zhang et al. demonstrated accurate, absolute temperature measurements using a small 129Xe surface coil and adiabatic 90° excitation in obese mice.7 The feasibility of 129Xe thermometry deep within the body and potential utility of spin-echoes8 are, however, not well established in the literature.
Purpose of this work was to develop a 129Xe chemical-shift imaging (CSI) pulse sequence employing both FID (parenchymal organs) and spin-echo acquisitions (fatty tissue, suppressing signals from rapidly exchanging compartments) using a volume coil and to investigate the potential for accurate thermometry in live rats by exploiting the very high temperature coefficient of the 129Xe lipid resonances.
Methods
A 2D CSI sequence was implemented according to the sequence diagram in Figure 1. A Shinnar–Le Roux refocussing pulse was designed (Matpulse,9 v6.0) using 128 steps, 2kHz bandwidth, pass-band ripple 1%, stop-band ripple 1%, 2ms duration. Table 1 shows sequence parameters.This study was conducted in accordance with the German animal welfare act (TierSchG) and approved by the Lower Saxony State Office for Consumer Protection and Food Safety (LAVES; 20/3501). 4 Lewis rats (2 males/2 females; Charles River Laboratories) were used in this study.
Imaging was performed at 3T (Vida, Siemens Healthcare) using a dual-resonant (123.2MHz/34.1MHz) circularly polarized birdcage coil (Rapid Biomedical). 129Xe was hyperpolarized (Polarean 9810) and dispensed into Tedlar bags (Jensen Inert Products). 129Xe was then drawn into a 500ml plastic syringe which was compressed using hydraulic actuation. 129Xe (~100ml/min) and oxygen (~400ml/min) with isoflurane (~2%) were spontaneously inhaled by the rats. Core body temperature was measured using a rectal temperature probe and controlled using a fluid heating system (Small Animal Instruments).
1H shimming was performed in the central abdomen and 1H spectra were acquired. 129Xe chemical shifts were water-referenced as previously described.10 129Xe CSI data were acquired with rat body temperatures ~37°C (measurement 1), ~34°C (measurement 2) and after reheating to ~37°C (measurement 3).
Lorentzians were fitted to spectra from regions of interest (ROIs) in the kidneys (FID, 4 Lorentzians) and abdominal fat (spin echo, 1 Lorentzian) to determine the lipid-dissolved 129Xe chemical shift. 129Xe temperature measurements were performed assuming a chemical shift of 191.8ppm at 37°C and a temperature coefficient of –0.21ppm/°C.7,10
Friedman tests were performed to test for longitudinal changes of 129Xe lipid chemical shift using a significance level of 0.05.
Results
Figure 2 shows exemplary imaging results in a 20 weeks old female rat weighing 246g for the initial measurement as well as ROIs for analysis of the kidneys and abdominal fat, respectively. Corresponding spectra are shown in Figure 3.A significant temperature-related change of chemical shift between measurements for both FID (p = 0.0498) and spin-echo acquisition (p = 0.0388) was found, see figure 4a,b. Figure 4c,d show scatter plots correlating 129Xe-MRI derived temperature measured in the local spectra with rectal temperature measurements. Bland–Altman plots for comparison of 129Xe temperature measurements with recal temperature are shown in figure 4e,f. Limits of agreement are narrower and mean absolute difference lower for spin-echo acquisitions.
Discussion
We developed a combined FID and spin-echo CSI sequence for intraabdominal non-invasive temperature measurements in free breathing rats. Local spectra from FID and spin-echo acquisitions clearly show strong shifts of lipid-associated resonances with temperature consistent with previous literature reports.The narrow, well-isolated lines in spin-echo CSI suggest that highly accurate and presumably also absolute temperature measurements can be made in vivo. Due to the spin-echo technique, more signal is obtained from fatty tissue. This is also supported by the narrow distribution of measurements for temperature and chemical shift observed in the scatter plots. Additionally, we could show, that FID signals could be used to measure temperature shifts in parenchymal organs like the kidney, however with wider absolute differences in the Bland–Altman plots.
While with the available hyperpolarization technology it is not foreseeable that the proposed method would be used clinically, it may be valuable as accurate reference standard for the development of 1H MRI thermometry or for the measurement of thermogenesis in brown-adipose tissue.11
A potential improvement would be the acquisition of 3D data for better structural delineation. This would likely require longer scan times and more efficient gas administration. Other limitations of this study are the low case number and potential temperature gradients within the rat.
Conclusion
This study demonstrates the feasibility of spatially resolved 129Xe MR thermometry in the rat‘s abdomen during free breathing. Accuracy and precision of in vivo 129Xe MRI temperature measurements could potentially be improved by employing spin-echo pulse sequences to suppress short T2 components with variable temperature shifts.Acknowledgements
The authors acknowledge Anja Standke‘s help with handling of laboratory animals.
References
1. Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 71, 209–249 (2021).
2. Chu, K. F. & Dupuy, D. E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer 14, 199–208 (2014).
3. Rieke, V. & Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging 27, 376–390 (2008).
4. Baron, P. et al. Influence of water and fat heterogeneity on fat‐referenced MR thermometry. Magn. Reson. Med. 75, 1187–1197 (2016).
5. Bomers, J. G. R. et al. MRI-guided focal laser ablation for prostate cancer followed by radical prostatectomy: correlation of treatment effects with imaging. World J. Urol. 35, 703–711 (2017).
6. Venkatesh, A. et al. Temperature Measurement Using the 129Xe Chemical Shift. in Proc. Intl. Soc. Mag. Reson. Med 9 2194 (2001).
7. Zhang, L. et al. Accurate MR thermometry by hyperpolarized 129 Xe. Magn. Reson. Med. 78, 1070–1079 (2017).
8. Virgincar, R. S. Preclinical Hyperpolarized 129 Xe MRI: Development, Applications, and Dissemination. (Duke University, 2018).
9. Matson, G. B. An integrated program for amplitude-modulated RF pulse generation and re-mapping with shaped gradients. Magn. Reson. Imaging 12, 1205–1225 (1994).
10. Antonacci, M. A., Zhang, L., Burant, A., McCallister, D. & Branca, R. T. Simple and robust referencing system enables identification of dissolved-phase xenon spectral frequencies. Magn. Reson. Med. 80, 431–441 (2018).
11. Branca, R. T. et al. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc. Natl. Acad. Sci. 111, 18001–18006 (2014).
Figures
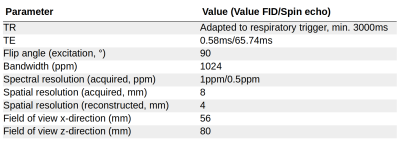
Table 1. Sequence parameters for 129Xe chemical shift imaging sequence.
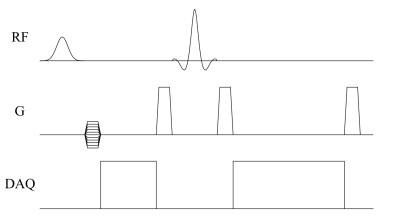
Figure 1. Sequence diagram of the FID and spin-echo 2D chemical-shift imaging sequence. Phase encoding is performed in two dimensions without slice selection. Magnetization is excited using a 440µs 90° Gaussian RF pulse and refocussed using a 2ms 180° Shinnar–Le Roux pulse.
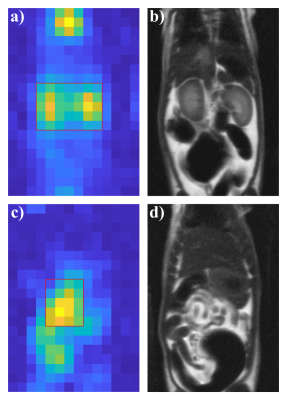
Figure 2. a) Maximum intensity projection of magnitude spectra over spectral domain shows signal in the FID acquisition consistent with perfusion. Both kidneys were selected for regional analysis. b) Coronal slice of 1H HASTE acquisition with matched in-plane field of view. c) Maximum intensity projection over data from spin-echo acquisition shows bright structures mainly consistent with visceral fat, d). Boxes show regions of interest (ROI) for local analysis, see Figure 3.
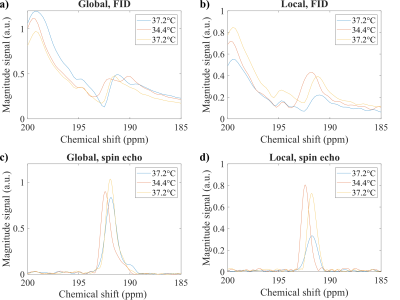
Figure 3. Spectra at different temperatures for measurement 1 (blue), 2 (orange) and 3 (yellow). a) FID spectra for all voxels seem crowded with resonances with different temperature shifts. b) FID spectra in the ROI (kidneys) from Figure 2a show relatively well isolated resonances near 192ppm with clear temperature shift. c) Global spin-echo spectra exhibit only resonances near 192ppm with consistent temperature shift, narrowed down in the spectra from the ROI (fat) in figure 2c, d).
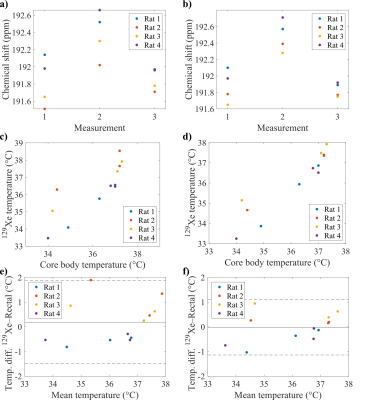
Figure 4. 129Xe in lipid chemical shifts obtained in local ROIs for the individual measurements in a) FID and b) spin-echo acquisitions. c), d) Scatter plots of 129Xe temperature and core body temperature measurements for FID and spin-echo acquisitions, respectively. e), f) Bland–Altman plots for measurements from rectal temperature probe and 129Xe temperature from FID and spin-echo acquisitions, respectively.