3538
pH-dependence of taurine 1H NMR chemical shifts: A potential method for pH determination and imaging1in-vivo MR, University Bremen, Bremen, Germany, 2Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
Synopsis
Magnetic resonance spectroscopy (MRS) allows non-invasive pH measurements via the chemical shift difference between a pH-dependent resonance and a reference. We studied the pH dependence of taurine chemical shifts in 1H MRS for a wide range of pH and temperature. At 37°C and in the physiological range (pH ~7.3), the chemical shifts show a bijective dependence on pH. This approach was evaluated by measurements on taurine phantoms using solutions with different pH and temperatures, demonstrating the possibility of absolute pH determination via the chemical shift of taurine.
Introduction
The pH value is an important marker for many disease processes and pathologies, including cancer and stroke. These pathologies result in changes in the intracellular pH (pHi) of the tissue. Thus, a non-invasive spatially and temporally highly resolved measurement of pHi can significantly contribute to the differentiation of healthy and diseased tissue. A conventional way of determining pHi in magnetic resonance spectroscopy (MRS) is measuring the chemical shift difference between a pH-dependent resonance and a reference 1.Prior knowledge on the chemical shifts and the J-coupling constants of metabolites is also of central importance when quantifying spectra based on established quantification programs that use a model function for each metabolite to minimize the number of variables during the fitting procedure 2-5. However, the accuracy of prior knowledge on the chemical shifts is essential for avoiding systematic quantification errors 6. In the course of a systematic investigation of the pH and temperature dependence of chemical shifts and coupling constants of important metabolites of brain metabolism, we have performed in vitro measurements on phantoms in a broad temperature and pH range. In particular, we have studied phantoms containing taurine (Tau) solutions with different pH values.Methods
(1) Measurements were performed on a wide-bore 400 MHz NMR spectrometer (Avance III HD electronics, Bruker Biospin, Germany) using a TXI NMR probe with a z-gradient. 1H spectra were acquired using a single-pulse sequence. The following sequence parameters were used: acquisition time 6.39 s, relaxation delay 15 s, spectral width SW = 8012 Hz, dummy scans 2 and number of accumulations NA = 16. Sample temperatures were adjusted to 40°C- 1°C.(2) A feasibility study was performed on a 9.4 T animal scanner (BioSpec 94/30 USR, AVANCE III, Bruker BioSpin, Ettlingen, Germany) equipped with a BGA‐12S HP B0 gradient system. RF excitations and signal detection were achieved with a quadrature birdcage coil with an inner diameter of 86 mm. Localized 1H spectra were acquired using a point resolved spectroscopy sequence (PRESS) with the following sequence parameters: echo time TE = 16.5 ms, repetition time TR = 2 s, NA = 128, SW = 4006 Hz, voxel size 8 × 8 × 8 mm3, acquisition time 4.16 min, and eddy current compensation using the unsuppressed water signal. Sample temperatures were adjusted to 37°C.
For the sample solutions taurine (10 mM) was dissolved in phosphate buffered saline (PBS). Additionally, (1) 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) (5 mM) or (2) N-acetylaspartate (NAA) (10 mM) were added as chemical shift references and the solutions were titrated to six pH values between 5.5-8.0.
Spectrum processing and determination of chemical shifts and coupling constants were performed using the software MestReNova 14.2.0 (Mestrelab Research S. L.). Data processing consisted of zero filling to 2048 K complex data points, Fourier transformation, and an automatic phase and baseline correction.
Results and Discussion
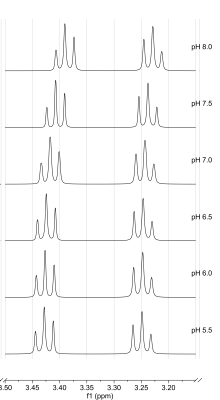
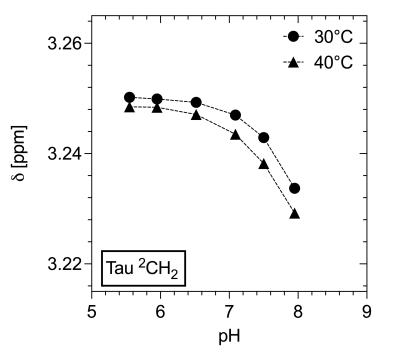
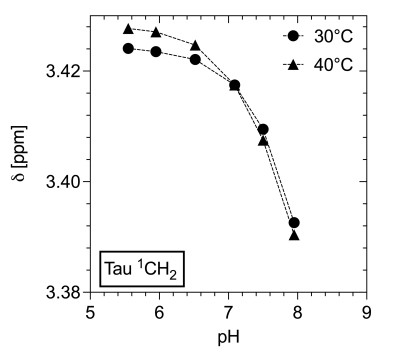
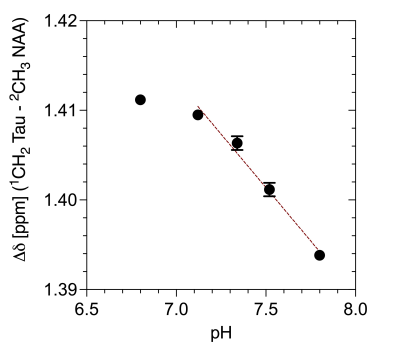
Figure 1 shows spectra with the resonances of Tau referenced to DSS (0 ppm) for 40°C as a function of pH. Both triplets show a downfield shift with more acidic pH. No changes in multiplet structure can be observed. The chemical shifts show a bijective dependence on pH in the physiological range. Additionally, it is important to note that the signals of the two proton groups shift differently (Figure 2, 3). The data recorded on the 400 MHz spectrometer has been supported by MRS data measured on the 9.4 T tomograph (Figure 4). Furthermore, the reproducibility is also shown (n=3), (line width of NAA 3 Hz). Assuming a linear regression in the physiologically interesting pH range of 7.1-8.0, a pH change of one-tenth would correspond to a chemical shift of 0.002 ppm (0.8 Hz, 9.4 T). The extent to which the accuracies can be achieved under in vivo conditions is under investigation.Conclusion
Both triplets of taurine show a rather large pH dependence, especially in the physiologically interesting range. Thus, in combination with MRS or MR spectroscopic imaging, this dependence provides a potential approach to determine pHi values absolutely and without the necessity of referencing to exogenous metabolites. Furthermore, the different pH dependence of the taurine triplets should be considered to avoid quantification errors if model functions of metabolite signals are used and pH may be reduced or increased.Acknowledgements
Deutsche Forschungsgemeinschaft, Grant/ Award Numbers: DR298/13-1, DR298/13-2, BO2467/4-1, BO2467/4-2, WE6575/1-1References
1. Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973; 248(20):7276–7278.
2. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993; 30:672–679.
3. Ratiney H, Coenradie Y, Cavassila S, et al. Time-domain quantitation of 1H short echo-time signals: background accommodation. Magn Reson Mater Phy. 2004; 16:284-296.
4. Ratiney H, Sdika M, Coenradie Y, et al. Time-domain semi-parametric estimation based on a metabolite basis set. NMR Biomed. 2005; 18:1-13.
5. Poullet JB, Sima DM, Simonetti AW, et al. An automated quantitation of short echo time MRS spectra in an open source software environment: AQSES. NMR Biomed. 2007; 20:493–504.
6. Wermter FC, Mitschke N, Bock C, et al. Temperature dependence of 1H NMR chemical shifts and its influence on estimated metabolite concentrations. Magn Reson Mater Phy. 2017; 30(6):579-590.
Figures