3531
Inductively Coupled Floating Coil for Hyperpolarized 13C Imaging of Mouse Brain1Department of Brain and Cognitive Sciences, McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Polarize ApS, Frederiksberg, Denmark, 3Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 5Department of Neurosurgery, Massachusetts General Hospital, Boston, MA, United States
Synopsis
We report an inductively coupled floating coil designed for hyperpolarized 13C MR spectroscopic imaging of mouse brain, with emphases on the simplicity of the circuit design, the ease of use, whole-brain coverage, and high SNR obtained. The floating coil is designed to “crown” the mouse head for a snug fit to achieve full coverage of the brain with good sensitivity. Here, we demonstrated the coil’s performance in normal mouse and glioblastoma multiforme mouse model at 4.7 T using a next-generation hyperpolarizer. High signal to noise ratio exceeding 70:1 was obtained in the brain with good spatial resolution (1.53mm x 1.53mm).
INTRODUCTION
Image resolution in MRI is limited by the signal to noise ratio (SNR) of the imaging system. Hyperpolarized (HP) 13C MR spectroscopic imaging (MRSI) is a promising technique to image enzyme-catalyzed metabolic transformations in vivo with >10,000 enhancement1. However, obtaining good spatial resolution in mouse brain is still a challenge due to its small size and non-replenishable HP-13C signals.Coil design that matches subject anatomy is critical for maximizing SNR. We have designed a simple 13C single-loop surface coil dedicated for mouse brain imaging. This inductively coupled floating coil2 closely fits the mid-point of mouse head and has whole-brain coverage with good sensitivity.
METHODS
The floating coil (Figure 1a) was made of 16gauge copper wire with a capacitor tuned to the 13C frequency of 50MHz at 4.7T. A variable capacitor was placed in parallel to allow a small tuning range to compensate for the load. The floating coil is coupled to a shielded loop (Figure 1b) which is connected to the in-system T/R switch, preamplifier, and RF power amplifier. This electrically balanced, untuned, pick-up loop3 was constructed from semi-rigid coaxial cable (UT-85C-FORM) on a 3D printed mechanical shuttle that was adjusted in position by a screw mechanism and was used to match to the system’s 50ohm impedance.The coil was placed like a crown on the head of a mouse in a prone position (Figure 1a). Motion of the head was constrained by ear and bite bars. The ear bars also helped to slightly lift the head so that the floating coil was not affected too much by respiratory motion of the body. We have observed the coil matching by reflective-power impedance measurements on a network analyzer over a minute to video changes in matching from animal breathing under isoflurane outside the scanner. This 13C coil was used in conjunction with a Bruker 1H volume coil.
Performance of this coil was first evaluated on a mouse with glioblastoma multiforme (GBM) tumor on its right hemisphere and we further improved the spatial resolution on normal mice. All animal procedures were performed under an IACUC-approved protocol. A sample of 18uL 13C-pyruvate with 30mM AH111501 was hyperpolarized in a d-DNP polarizer (Polarize SpinAligner, Frederiksberg, Denmark). Dissolution was performed by using 3.2mL of dissolution medium (Trizma, NaCl, NaOH and EDTA), yielding 80mM 13C-pyruvate solution for injection. Chemical shift imaging (CSI) started 10s after the start of injection.
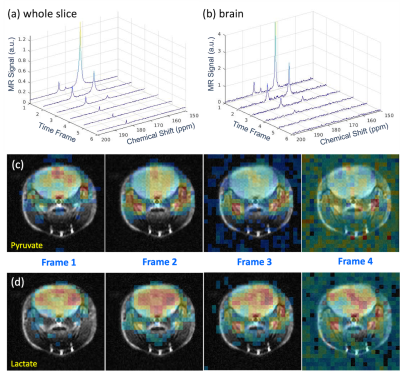
Coronal CSI was acquired on the GBM model, following a bolus injection of ~200uL of HP [2-13C]pyruvate, with 30mm x 24mm field of view, 12 x 10 matrix size, 2.5mm x 2.4mm resolution, 8mm slice, 1.08ms/142.5ms TE/TR, 10° constant flip-angle, and centric phase encodings. Axial CSI was collected on a normal mouse with a similar CSI protocol but better in-plane resolution of 1.53 mm x 1.53 mm and a thinner slice (4 mm). The 15s CSI was repeated 6 times following a bolus injection of ~120uL of HP [1-13C]pyruvate. Anatomical images were acquired using T2 RARE. CSI data were analyzed using a custom software in Matlab (30Hz line-broadening and zero fill once on all dimensions).
RESULTS
Good matching of -30dB at 50.38MHz was obtained on mouse heads and confirmed on the scanner (Figure 1c). The coil matching video showed small fluctuations within 2% due to respiratory motion.Liquid-state polarization was 60% ± 9% for [1-13C]pyruvate and ~5% less for [2-13C]pyruvate. T1 relaxation time of [1-13C]pyruvate in a syringe was 75 s at 4.7 T.
Coronal CSI of the GBM model showed large SNR, good image quality and elevated [2-13C]lactate signal on the tumor (Figure 2). Given the high polarization, we were able to improve the spatial resolution to 9.4μL voxel size in the axial CSI of normal brain (Figure 3). Most of the pyruvate signals were in the vasculature, as expected. Large [1-13C]lactate signals lasted in the brain for about a minute. SNR of [1-13C]lactate averaging over 6 frames was 71:1 in the brain and 83:1 in the vessels. SNR of [1-13C]pyruvate was 163:1 in the brain and 283:1 in the vessels.
DISCUSSION
We have shown a very simple 13C coil that can be made on the bench with a small budget to achieve good SNR and high image quality of mouse brain using basic CSI acquisition. Acquisition efficiency can be further improved with more efficient MRSI techniques, such as EPSI or Spiral-CSI, and better temporal resolution of a few seconds per frame is conceivable with these sequences. The high polarization from SpinAligner attributed significantly to the high SNR. However, the SNRs from this coil are still remarkable even after dividing the SNRs by 2 or 3 to account for the high polarizer. This coil is limited by homogeneous RF excitation (B1+). Similar ideas of the inductively coupled coil design can be applied to other coil geometries to improve the B1+ homogeneity.CONCLUSION
High quality images of 13C-pyruvate and its metabolite 13C-lactate were obtained from mouse brain with good spatial resolution and good sensitivity using an inductively coupled floating coil tailored for HP-13C MRSI of mouse brain. This coil is simple to make and easy to use. The design concept can be potentially applied to other coil geometries.Acknowledgements
This work was supported by NIH funds S10OD021569, S10OD021768, R21GM137227, R01EB029829, R01CA227821, and Seeman Family MGH Research Scholarship in Neuro-Oncology.
References
1. Ardenkjær-Larsen JH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100(18):10158-63. doi: 10.1073/pnas.1733835100.
2. J Mispelter, M. Lupu and A Briguet. NMR Probeheads for Biophysical and Biomedical experiments. 2nd edition; Imperial College Press; 2015; p226.
3. J Mispelter, M. Lupu and A Briguet. NMR Probeheads for Biophysical and Biomedical experiments. 2nd edition; Imperial College Press; 2015; p656.
Figures
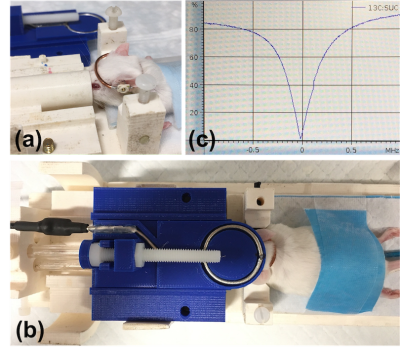
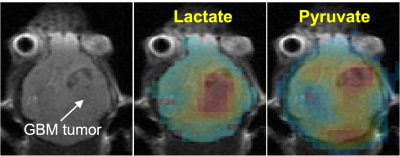
Figure 2: [2-13C]pyruvate and [2-13C]lactate images (in colors) of a GBM mouse model (tumor indicated by the arrow) superimposed on an anatomical T2-weighted image. Acquired voxel size was 48 μL.