3530
Feasibility of the Ventilatory ADC Approach Using Hyperpolarized 129Xe Pulmonary MRI1Physics and Astronomy, The University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3School of Biomedical Engineering, Faculty of Engineering, The University of Western Ontario, London, ON, Canada
Synopsis
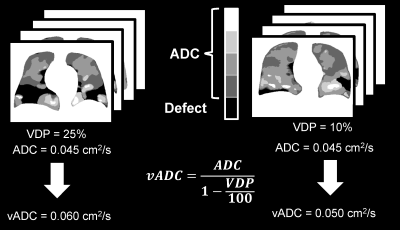
Hyperpolarized 129Xe lung MRI is an efficient technique used to investigate and assess pulmonary diseases. However, the longitudinal observation of the emphysema progression using hyperpolarized gas MRI-based ADC can be problematic, as the disease-progression can lead to increasing unventilated-lung areas, which likely excludes the largest ADC estimates. One solution to this problem is to combine static-ventilation and ADC measurements following the idea of 3He MRI ventilatory ADC (vADC). We have demonstrated this method adapted for 129Xe MRI to help overcome the above-mentioned shortcomings and provide an accurate assessment of the emphysema progression.
Purpose
Hyperpolarized gas pulmonary MRI1,2 provides physiologically relevant biomarkers of obstructive lung disease including emphysema, bronchopulmonary dysplasia, congenital lobar emphysema and alpha-1 antitrypsin deficiency.3-5 However, the longitudinal observation of the emphysema progression using hyperpolarized gas MRI-based ADC can be problematic, as the disease progression can lead to increasing unventilated lung areas, which likely excludes the largest ADC estimates. This can result in underestimation of the global mean ADC values, masking the emphysema severity. Clearly, the static-ventilation measurements providing the gas-distribution should still show an increase in the ventilation-defects reflecting emphysema-progression, but these measurements do not provide the quantitative information about the lung parenchyma microstructure. One solution to this problem is to combine static-ventilation and ADC measurements following the idea of ventilatory ADC (vADC).6 The feasibility of this approach has been recently demonstrated using the 3He static-ventilation and diffusion-weighted data.6 We hypothesize that this method adapted for 129Xe MRI should help to overcome the above-mentioned shortcomings and provide an accurate assessment of the emphysema-progression. For this work, we used the static-ventilation and ADC data acquired using 129Xe MRI in a small group of study subjects to demonstrate the feasibility of the xenon ventilatory ADC approach as a potential clinical-tool the longitudinal observation and evaluation of emphysema-progression.Methods
Five patients with written informed consent provided to an ethics-board-approved study protocol, underwent spirometry and 1H/129Xe MRI scanning. 129Xe imaging was performed at 3.0T (MR750, GEHC, WI) using whole-body gradients (5G/cm maximum) and a commercial 129Xe quadrature-flex RF coil (MR Solutions, USA).8 129Xe static-ventilation-images were acquired using a coronal plane 3D FGRE sequence (TE/TR/initial-flip-angle=1.5ms/5.1ms/1.3o, variable-flip-angle,7 Bandwidth=16kHz, reconstructed matrix size=128x128x16, and FOV=40x40x24cm3, voxel size=3x3x15mm3) as previously described.8 All images were acquired in breath-hold (<16sec) after inspiration of 1.0L of gas (129Xe/4He mixture, 30/70) from functional-residual-capacity. Pre- (baseline) and post-salbutamol data sets were acquired for each study subject. Hyperpolarized 129Xe gas (polarization=35%) was obtained from a turn-key, spin-exchange polarizer system (Polarean-9820 129Xe polarizer).4 1H MRI was performed as previously described.9 Image SNR was calculated for the central slices for each coronal view by selecting two regions of interest with similar size, one inside the lung with homogeneous signal and one outside the lung containing noise.10 VDP was generated using a semi-automated segmentation algorithm as previously described.8 In all xenon measurements the diffusion-sensitization gradient pulse ramp up/down time=500μs, constant time=2ms, ΔXe=5.2ms, providing two b-values 0, and 12.0s/cm2. A multi-slice interleaved (two interleaves) centric 2D FGRE diffusion-weighted sequence was acquired for seven 30mm coronal slices (TE=10msec, TR=13msec, reconstructed matrix size=128x128, and FOV=40x40cm2, constant-flip-angle=4o, 14sec breath-hold). Matching static-ventilation images with 3x3x15mm3 voxel-size were obtained by transforming the 2D multi slice ADC k-space into the 3D ADC k-space, then sandwiching the empty coil ADC k-space and one xenon ADС signal k-space (so the total number of slice was 14 per b-value) and performing a 3D Fast Fourier Transform starting with the z-direction following the key-hole technique method.11,12 The ADC (b=0/b=12s/cm2) maps were generated as previously described13,14 for all xenon measurements. Calculated ADC values were normalized on the corresponding VDP-estimates to obtain vADC as it is shown on Fig.1 as previously described.6Results
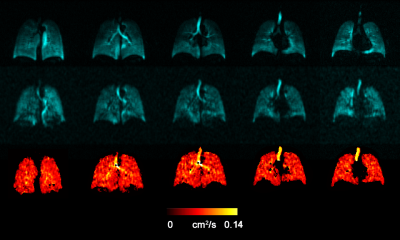
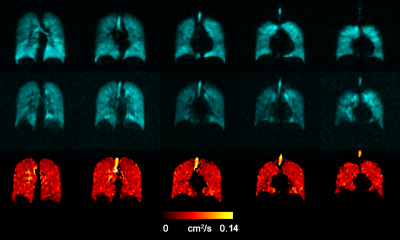
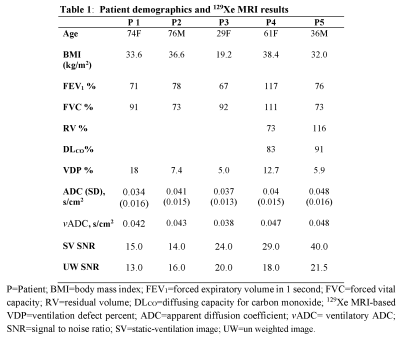
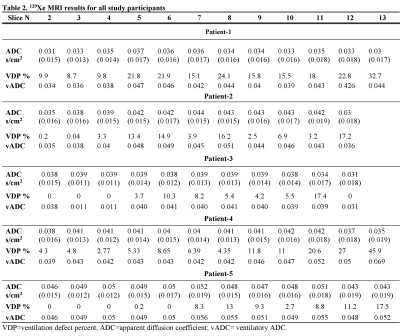
Fig.2 and 3 show the acquired static-ventilation images (top-panel), matched voxel-size unweighted (b=0,) images (middle-panel) and correspondent ADC maps (bottom-panel) in coronal view for two patients demonstrating a good match between static-ventilation and key-hole-based unweighted slices. Table 1 summarizes demographic and PFT-information, as well as imaging results including SNR (central slice) for static-ventilation and matched voxel-size unweighted images, global mean VDP, and global mean ADC/vADC for all patients. The calculated mean SNR values across all xenon images ranged between 10 and 35. Table 2 shows the slice by slice VDP, ADC, and vADC estimations for all participants. The generated global mean VDP estimates for the study subjects were between 5% and 18%. The generated global mean ADC/(vADC) estimates for the study subjects were between 0.034s/cm2/(0.034s/cm2) and 0.048s/cm2/(0.048s/cm2).Discussion and Conclusion
In this proof-of-concept-study, we showed that the emphysema-progression can be potentially quantified with using the pulmonary static-ventilation and diffusion-weighted images of hyperpolarized 129Xe utilizing the ventilatory ADC approach. The study results suggest that the diffusion data reconstructed with the key-hole-technique had sufficient SNR to generate reliable ADC maps and reasonable matching with the static-ventilation data. A rigid and more homogenous coil15 combined with a phased-receive-array16 could substantially improve the ADC data quality and potentially replace the isotopically-enriched 129Xe with the natural-abundant xenon,17 and consequently, reduce the cost of 129Xe MRI for patients. For the first time the feasible of the ventilatory 129Xe ADC approach was demonstrated and shown that this method can be used to accurately evaluate the emphysema progression. This is important in the light of the FDA approval16 for 129Xe MRI. Furthermore, this increases the opportunity for clinical-translation of using 129Xe lung MRI as a tool for better treatment of patients with acute and chronic-lung-disease. For future work, we plan rescan the study participants in twelve months and normalize the 129Xe ADC by 129Xe VDP for an accurate assessment of the emphysema progression over the year-interval.Acknowledgements
We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada, R5942A04, and a Western University Research Catalyst Grant.References
1 Mugler, J. P., 3rd & Altes, T. A. Hyperpolarized 129Xe MRI of the human lung. J Magn Reson Imaging 37, 313-331, doi:10.1002/jmri.23844 (2013).
2 Driehuys, B. et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology 262, 279-289, doi:10.1148/radiol.11102172 (2012).
3 Kirby, M. et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 265, 600-610, doi:10.1148/radiol.12120485 (2012). 4 Kaushik, S. S. et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65, 1154-1165, doi:10.1002/mrm.22697 (2011).
5 Kirby, M. et al. Hyperpolarized 3He and 129Xe magnetic resonance imaging apparent diffusion coefficients: physiological relevance in older never- and ex-smokers. Physiol Rep 2, doi:10.14814/phy2.12068 (2014).
6 Westcott, A., Capaldi, D. P. I., Ouriadov, A., McCormack, D. G. & Parraga, G. Hyperpolarized (3) He MRI ventilatory apparent diffusion coefficient of alpha-1 antitrypsin deficiency. J Magn Reson Imaging 49, 311-313, doi:10.1002/jmri.26202 (2019).
7 Ouriadov, A. V., Lam, W. W. & Santyr, G. E. Rapid 3-D mapping of hyperpolarized 3He spin-lattice relaxation times using variable flip angle gradient echo imaging with application to alveolar oxygen partial pressure measurement in rat lungs. MAGMA 22, 309-318, doi:10.1007/s10334-009-0181-3 (2009). 8 Svenningsen, S. et al. Hyperpolarized (3) He and (129) Xe MRI: differences in asthma before bronchodilation. J Magn Reson Imaging 38, 1521-1530, doi:10.1002/jmri.24111 (2013).
9 Kirby, M., Pike, D., Coxson, H. O., McCormack, D. G. & Parraga, G. Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology 273, 887-896, doi:10.1148/radiol.14140161 (2014).
10 Dominguez-Viqueira, W., Ouriadov, A., O'Halloran, R., Fain, S. B. & Santyr, G. E. Signal-to-noise ratio for hyperpolarized (3)He MR imaging of human lungs: a 1.5 T and 3 T comparison. Magn Reson Med 66, 1400-1404, doi:10.1002/mrm.22920 (2011).
11 Niedbalski, P. J. et al. Mapping and correcting hyperpolarized magnetization decay with radial keyhole imaging. Magn Reson Med 82, 367-376, doi:10.1002/mrm.27721 (2019).
12 Ranota, T. K., Serrai, H., McCormack, D. G., Parraga, G. & Ouriadov, A. Feasbility of Single Breath-hold Isotropic Voxel 129Xe MRI in COVID-19 Survivors using a Key-Hole Method [abstract]. ISMRM 30th Annual Meeting (2021).
13 Abascal, J. F. P. J., Desco, M. & Parra-Robles, J. Incorporation of prior knowledge of the signal behavior into the reconstruction to accelerate the acquisition of MR diffusion data. ArXiv e-prints 1702 (2017). <http://adsabs.harvard.edu/abs/2017arXiv170202743A>.
14 Ouriadov, A., Lessard, E., Sheikh, K., Parraga, G. & Canadian Respiratory Research, N. Pulmonary MRI morphometry modeling of airspace enlargement in chronic obstructive pulmonary disease and alpha-1 antitrypsin deficiency. Magn Reson Med 79, 439-448, doi:10.1002/mrm.26642 (2018).
15 Farag A, Wang J, Ouriadov A, Parraga G & G., S. Unshielded and asymmetric RF transmit coil for hyperpolarized 129Xe human lung imaging at 3.0 T. In Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia, 1233 (2012).
16 Chang, Y. V., Quirk, J. D. & Yablonskiy, D. A. In vivo lung morphometry with accelerated hyperpolarized (3) He diffusion MRI: a preliminary study. Magn Reson Med 73, 1609-1614, doi:10.1002/mrm.25284 (2015).
17 Stewart, N. J., Norquay, G., Griffiths, P. D. & Wild, J. M. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med 74, 346-352, doi:10.1002/mrm.25732 (2015).
Figures