3527
A 31P MRS study of Target Engagement of Terazosin in Neurodegenerative Diseases
Jia Xu1, Sarah E Ernst2, Erin E Reasoner3, Stephen A Cross3, Alivia N Brinker3, Cameron A Keomanivong4, Zach Elias4, Daniel R. Thedens1, Nandakumar Narayanan4, Jordan L Schultz3, Michael J Welsh2, and Vincent A Magnotta1
1Radiology, University of Iowa, Iowa City, IA, United States, 2Internal Medicine, University of Iowa, Iowa City, IA, United States, 3Psychiatry, University of Iowa, Iowa City, IA, United States, 4Neurology, University of Iowa, Iowa City, IA, United States
1Radiology, University of Iowa, Iowa City, IA, United States, 2Internal Medicine, University of Iowa, Iowa City, IA, United States, 3Psychiatry, University of Iowa, Iowa City, IA, United States, 4Neurology, University of Iowa, Iowa City, IA, United States
Synopsis
Terazosin is an FDA-approved medicine that has been used primarily for men and data for women are missing. For the first time, the target engagement of Terazosin in neurodegenerative diseases was assessed for both sexes. We chose the βATP/Pi ratio as a biomarker to study the brain energy metabolism and found strong correlations between the in vivo brain ATP levels to whole blood ATP levels. We observed clear sex differences in response to different TZ doses in both mice and humans. Our data suggest that the optimal dose for females may need to be lower as compared to males.
Introduction
Impaired brain energy metabolism is a common feature of neurodegenerative diseases such as Parkinson’s disease (PD) and Huntington's disease (HD). Recently, Terazosin (TZ), an FDA-approved medicine that is used to treat benign prostate hypertrophy, was found to increase brain ATP levels by stimulating phosphoglycerate kinase 1 (PGK1) activity, an ATP-generating enzyme in glycolysis1. Human clinical databases also reveal that TZ may be neuroprotective in PD1. However, the current TZ dose was based on treating benign prostatic hypertrophy, the optimal TZ dose to increase brain ATP in both sexes is still unknown2. 31P MRS has the unique advantage of directly monitoring precise details of energetic metabolites like ATP and inorganic phosphate (Pi). Here we assessed the target engagement of TZ of both sexes in mice and humans at varying doses by 31P MRS at 7T.Methods
Mice: 30 mice were divided into 6 groups and treated with varying TZ doses (0, 0.1, 1, 10, 100, 1000 μg/kg/d) by IP injection. Mice were scanned before and after receiving their respective dose of TZ for 14 days. During scanning, the mice were anesthetized with isoflurane. The body temperature of the mice was maintained with heated air, and respiration rate was monitored. The mice MRS were collected on a GE 7T small animal scanner (MR901) with a 1H/31P dual tuned RAPID biomedical surface coil. The animal studies were approved by the IACUC of our institution. Human: 34 participants (age: 58±15 years) were recruited for both MRI and blood draws. 14 patients with neurodegenerative diseases (9 HD, 5 PD) and 20 sex- and age-matched healthy controls were imaged on a GE MR950 7T scanner. The MR session included the following scans: 1) a localizer, 2) 1H anatomical image, 3) high order B0 shimming including three linear and five second-order terms, 4) Bloch-Siegert estimation of center frequency and transmitter gain, and 5) quantitative non-localized 31P MRS using a free induction decay (FID) sequence. The metabolites of interest were defined with AMARES and fixed boundaries2,3. The peak area was calculated simply as the summation of all data points within the boundaries. The defined ranges were sufficiently broad that the peak area could be reliably calculated even with variations in peak locations due to differences in pH2,3. The human scan was approved by the IRB of our institution.Results
We compared the TZ-induced 31P MRS metabolite ratios to the whole blood ATP levels from the same participants and found that the changes in the two assays were correlated (Fig. 2). Data from the mouse study suggests that the optimal TZ dose to increase brain ATP levels is 1.0 μg/kg/d for males, but 0.1 μg/kg/d for females. The sex difference in TZ dose-response is also observed in human participants. As shown in Fig. 4, the 14 patients with PD or Huntington’s disease (HD) respond differently to TZ (p=0.08). The female participants had a greater ATP response to a dose of 1.0 mg while the male greatest increase in ATP levels was at 5.0 mg (p=0.02). We did not observe a significant increase in ATP levels at either TZ dose in the control subjects.Discussion
TZ is a drug that has been used primarily for men and data from women are missing. For the first time, we assessed the target engagement of TZ for both sexes with animal and human models. We found the non-invasive brain 31P MRS significantly correlates with the whole blood ATP assay. This correlation suggests that 1) both assays may capture TZ-induced ATP changes; 2) The TZ-induced changes cross the blood-brain barrier; 3) 31P MRS metabolite ratios can be used as biomarkers for assessing target assessment of TZ. We chose the βATP/Pi ratio as a biomarker of ATP levels to exclude possible contaminations from ADP or NADH signals. We observed similar sex differences in the response to TZ dose in both mouse and human models. We found male participants with a neurodegenerative disorder had the greatest ATP increase with a 5 mg TZ dose, which is consistent with the previous finding that PD patients taking TZ to treat enlarged prostate had slower PD progression and reduced PD complications1. However, the female participants with a neurodegenerative disorder had a larger response at a dose of 1 mg. It is possible that this difference is due to body mass differences between the sexes. A major limitation of this ongoing project is the small sample size: there are only 2 or 3 mice for each sex at each dose, and there are only 14 patients and 20 healthy controls. Despite this limitation, our data provide useful information that may help to guide dose selection for future clinical trials.Conclusion
We found the dose-response to TZ was different in males as compared to females. The optimal dose of TZ to increase brain ATP levels are 5 mg for male participants with a neurodegenerative disorder. In contrast, the female participants with a neurodegenerative disorder had a greater increase in ATP levels at 1.0 mg. It is possible that this difference is due to body mass differences between the sexes.Acknowledgements
This work is supported by Michael J. Fox Foundation, the National Institutes of Health (UL1TR002537), and the Carver Foundation. Imaging in this study was conducted on equipment supported by S10RR028821.References
- Cai, R.; Zhang, Y.; Simmering, J. E.; Schultz, J. L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P. M.; Narayanan, N. S.; Yuan, Y.; Chen, Z.; Su, W.; Han, Y.; Zhao, C.; Gao, L.; Ji, X.; Welsh, M. J.; Liu, L., Enhancing glycolysis attenuates Parkinson's disease progression in models and clinical databases. J Clin Invest 2019, 129 (10), 4539-4549.
- Schultz, J., A Pilot to Assess Target Engagement of Terazosin in Parkinson’s Disease. Parkinsonism & Related Disorders in press.
- Xu, J.; Schulte, R. F.; Kearney, W., R; Magnotta, V., A, Precision and Reliability of Metabolite Quantification of 31P MRS at 7T. Proc. Intl. Soc. Mag. Reson. Med. 2021, (29), 1787.
Figures
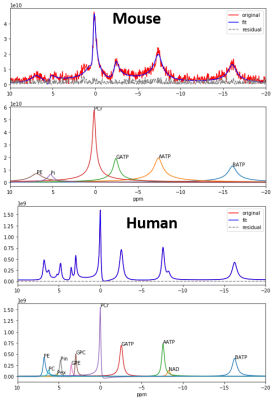
Fig. 1. Representative spectra of 31P
MRS of mouses and human
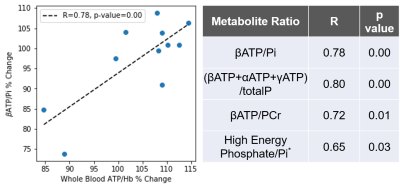
Fig. 2. The TZ-induced
changes in metabolite ratios such as βATP/Pi
correlate with the changes in whole blood ATP.
*High Energy Phosphate refers to (βATP+αATP+γATP+PCr).
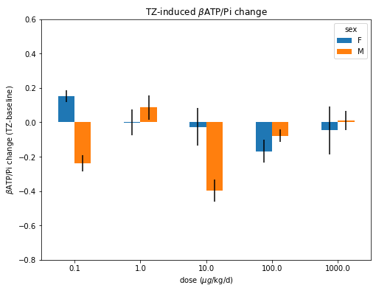
Fig 3. βATP/Pi change in male and female mouse
brains at different doses of TZ.
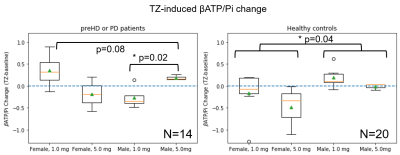
Fig. 4. Human response to 1.0 mg and 5.0
mg TZ assessed by βATP/Pi change. Dashed lines at 0 represent no change. Left: Male and female patients (pre-HD or PD patients) taking 1.0 and 5.0 mg
TZ, respectively. (p=0.08 by 1-way ANOVA). The male participants taking 5.0 mg are significantly different from those taking 1.0 mg (p=0.02 by Student’s t-test). Right: Sex- and age-matched healthy
participants taking 1.0 and 5.0 mg TZ, respectively. The male participants
taking both 1.0 and 5.0 mg TZ are significantly different from the females (p=0.04
by Student’s t-test).
DOI: https://doi.org/10.58530/2022/3527