3526
Development of Hyperpolarized 15N-labeled Metronidazole as an MR Contrast Agent by d-DNP Technique: A Potential Antibiotic and Hypoxia Probe
David Guarin Bedoya1,2, Erin Elizabeth Hardy1, Anna Samoilenko3, Sameer Joshi3, Jason Stockmann1, Jan Henrik Ardenkjaer-Larsen2,4, Matthew S. Rosen1,5,6, Boyd M. Goodson7, Thomas Theis8, Eduard Y Chekmenev3,9, and Yi-Fen Yen1
1Radiology Department, Athinoula A. Martinos Center for Biomedical Imaging, Massachusttes General Hospital, Boston, MA, United States, 2Polarize ApS, Frederiksberg, Denmark, 3Department of Chemistry, Wayne State University, Detroit, MI, United States, 4Department of Electrical Engineering, Technical University of Denmark, Kongens Lyngby, Denmark, 5Radiology Department, Harvard Medical School, Boston, MA, United States, 6Physics Department, Havard University, Boston, MA, United States, 7School of Chemical and Biomolecular Sciences, Southern Illinois University, Carbondale, IL, United States, 8Department of Chemistry, North Carolina State University, Raleigh, NC, United States, 9Russian Academy of Sciences, Moscow, Russian Federation
1Radiology Department, Athinoula A. Martinos Center for Biomedical Imaging, Massachusttes General Hospital, Boston, MA, United States, 2Polarize ApS, Frederiksberg, Denmark, 3Department of Chemistry, Wayne State University, Detroit, MI, United States, 4Department of Electrical Engineering, Technical University of Denmark, Kongens Lyngby, Denmark, 5Radiology Department, Harvard Medical School, Boston, MA, United States, 6Physics Department, Havard University, Boston, MA, United States, 7School of Chemical and Biomolecular Sciences, Southern Illinois University, Carbondale, IL, United States, 8Department of Chemistry, North Carolina State University, Raleigh, NC, United States, 9Russian Academy of Sciences, Moscow, Russian Federation
Synopsis
In this work, we developed a DNP hyperpolarized 15N-labeled metronidazole contrast agent. The formulation of the sample and the DNP process were optimized to reduce the build-up time constant down to 22 min while reaching liquid state signal enhancements of 104. The T1 relaxation times were measured in liquid-state, and found to be minutes long. Due to the high enhancement and long T1, MNZ hyperpolarized 15N signal could be monitored for several minutes. The contrast agent was tested with MRS in-vivo, in a rat brain. The hyperpolarized MNZ signal remained above the noise level for more than a minute.
Intoduction
Although the d-DNP technique has been used to develop hyperpolarized 15N-labeled contrast agents1,2, the applications of 15N d-DNP remain scarce due to the low 15N MR sensitivity2,3 and limited range of molecular probes with metabolic relevance. Additionally, the achievable polarization of 15N sites with DNP is normally limited to 1-2% and requires several hours of hyperpolarization2,4. Here, we used d-DNP to hyperpolarize and validate [1,2,4-15N]metronidazole ([15N3]MNZ). [15N3]MNZ has been hyperpolarized with the SABRE-SHEATH technique4, but this hyperpolarization technique has not been translated to in vivo yet. The goal of this project is to utilize the characteristically long hyperpolarization lifetime (i.e. T1 relaxation time) of 15N sites of [15N3]MNZ (up to ten minutes4) of the next-generation d-DNP hyperpolarizer to evaluate its potential for an in vivo hyperpolarized contrast agent. Besides the particularly long hyperpolarization lifetime of MNZ 15N sites, the MNZ molecule is of major biological interest. MNZ is an FDA-approved antibiotic member of the family of nitroimidazoles, able to act in a wide range of anaerobic bacterial infections. Metronidazole is safe and well tolerated in large doses including intravenous administration. The reduction of the nitroimidazoles family under hypoxic conditions makes them ideal probes for hypoxia, as has been shown in the literature5,6.Method
All samples were hyperpolarized on a SpinAligner 7 equipped with a broadband DNP probe with an RF circuit tunable to 13C, 15N, and other nuclei with Larmor frequencies in between. The dissolution experiments were performed by dissolving the DNP samples into 4 mL of Trizma buffer (100 mM, pH = 7.6). The samples were transferred to a 4.7 T Bruker pre-clinical scanner, with a transfer time of ~25 s. A homemade surface coil tuned to 15N Larmor frequency was used to perform the dynamic spectroscopy experiments.To develop and validate hyperpolarized [15N3]MNZ, the sample composition and the DNP parameters were empirically optimized. To this end, the signal build-up was monitored during the hyperpolarization process, Figure 1. Different samples containing [15N3]MNZ were tested with different radical concentrations and compositions. In order to reach 1.5 M concentration of MNZ, sample compositions with almost 100% DMSO concentration were required.
The liquid-state polarization and T1 of the different 15N sites were estimated by performing a dynamic spectroscopy experiment on a syringe containing the DNP sample after dissolution. The polarization decay due to the excitation pulses was considered during the T1 estimation.
Results
A dynamic spectroscopy experiment was performed on a syringe, and the resulting spectrum is displayed in Figure 2. A series of 20o hard excitation pulses, on a time intervalley of 3 s were used to acquire the pseudo-2D spectrum. After integrating the different resonances, the resulting estimated relaxation times for 15N1, 15N2 and 15N4 , and were found to be 157±1.9 s, 580.9±3.2 s, and 30.5±1.13 s respectively. The signal enhancement and polarization evolution are displayed for the different 15N resonances in Figure 3.The hyperpolarized [15N3]MNZ was also tested in-vivo. The same dynamic spectroscopy experiment used for the syringe run was conducted on a rat brain. For this experiment the surface coil was located on top of the rat's brain. After dissolution and transfer, 2 mL of the hyperpolarized sample were injected over 20 s into the rat tail vein. In the resulting pseudo-2D spectrum, the estimated T1 values were 59 s for 15N1 site and 44 s for 15N2 site respectively. The resulting spectrum and the signal evolution of 15N1 and 15N2 resonances are displayed in Figure 4.
Discussion
In the syringe run, the signal of [15N3]MNZ 15N resonances remains above the noise level for several minutes (figure 2) and the achieved signal enhancements are in the order of 104 at 4.7 T--unprecedented values for 15N hyperpolarization with DNP. The good signal enhancement and long T1 relaxation times allowed us to detect the 15N signal of the different MNZ 15N sites in-vivo. The signal in-vivo is found to be lower than the signal of HP [15N3]MNZ in the syringe, likely due to biological T1 decrease in vivo. Nevertheless, the resonances of 15N1, and 15N2 can be observed for more than a minute (figure 4). The resulting spectrum shows that a substantial amount of the injected HP [15N3]MNZ was delivered to the brain after circulating in the body.Conclusion
We have hyperpolarized 15N-labeled MNZ with DNP for the first time. The optimization of the sample formulation and the DNP parameters helped to reduce the polarization buildup time constant to ~22 min and achieve remarkable enhancements compared with the reported values on other 15N DNP probes. The hyperpolarized signals of the 15N resonances in [15N3]MNZ lasted for several minutes in a syringe, at 4.7 T, and over a minute in-vivo, indicating the potential for kinetic studies of its reduction reactions in hypoxic conditions or as antibiotic in cell work and in animal models. We are currently developing a cerebral hypoxia model in rats to test the utility of this new molecular probe in-vivo.Acknowledgements
This work was supported by NIH funds S10OD021768, R21GM137227, R01EB029829. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
- Srkhar, R. et al. Proton NMR of 15N-choline metabolites enhanced by dynamic nuclear polarization, J Am Chem Soc, 2009 131, 16014-16015
- Chiavazza, E. et al. 15N-permethylated amino acids as efficient probes for MRI-DNP applications. Contrast Media Mol. Imaging, 2013, 8, 417-421.
- Gamliel, A. et al. Hyperpolarized [15N]nitrate as a potential long lived hyperpolarized contrast agent for MRI, 2019, 299, 188-195.
- Shchepin R. et al. Hyperpolarizing Concentrated Metronidazole 15NO2 Group Over Six Chemical Bonds with More Than 15% Polarization and 20 Minute Lifetime. Chem Eur J. 2019;25:8829-8836.
- Upcroft, P. et al. Drug targets ans mechanisms of resistance in the anaerobic protozoa Clin. Microbiol. Rev. 2001, 14, 150
- Kizaka-Kondoh, SH. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia Cancer Sci. 2009, 100, 1366-1373.
- Ardenkjær-Larsen, JH. et al. Cryogen-Free dissolution Dynamic Nuclear Polarization polarizer operating at 3.35 T, 6.70 T and 10.1 T. Magnetic Resonance in Medicine. 2019, 8, 2184-2194.
Figures
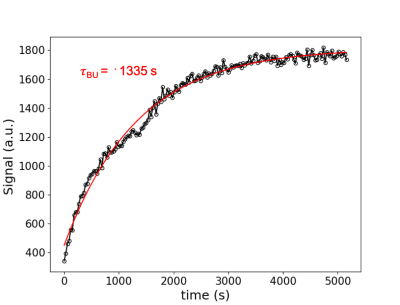
Figure 1: 15N solid-state signal buildup during hyperpolarization. Signal monitored with small tip angle excitation pulses every 30 s. Signal effectively reaches maximum after ~50 min of hyperpolarization. In order to extract the build-up constant time  , the data was fitted to the following equation describing the evolution of the magnetization along the z-axis: M = A*(1-exp(-t/tau)) + B
, the data was fitted to the following equation describing the evolution of the magnetization along the z-axis: M = A*(1-exp(-t/tau)) + B
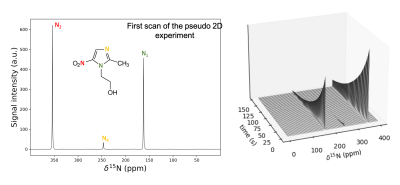
Figure 2: Left: first scan of the pseudo-2D spectrum obtained from the syringe following the d-DNP experiment. Right: stacked plot of the pseudo-2D spectrum; the signals from the MNZ 15N sites are observable for several minutes.
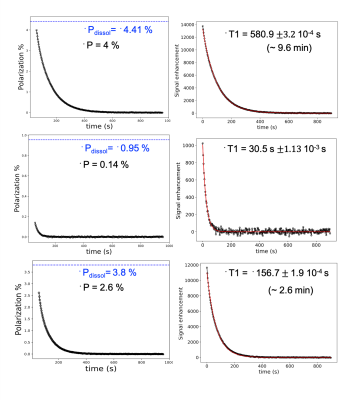
Figure 3: Time evolution of polarization and signal enhancement of 15N2 (top), 15N4 (middle), and 15N1 (bottom). Left: polarization evolution of the 15N resonances; in blue is the extrapolated value of the polarization at the moment of the dissolution experiment. Right: signal enhancement evolution fitted to an exponential decay to extract the T1.
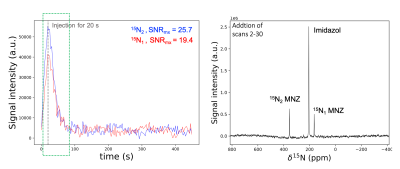
Figure 4: Left: signal evolution of the 15N1-2 MNZ resonances with the respective maximum SNR. Right: sum of the scans 2-30, duration denoted by the green square in the left figure. A phantom containing thermally-polarized [15N2]imidazole solution at a 7 M concentration was placed to the other side of the surface coil to be used as chemical shift and signal intensity reference.
DOI: https://doi.org/10.58530/2022/3526