3525
A Dissolved-Phase 129Xe Phantom for Quantitative Gas-Exchange MRI1Center for Pulmonary Imaging Research, Division of Pulmonary Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Department of Biomedical Engineering, University of Cincinnati, Cincinnati, OH, United States, 3Imaging Research Center, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 4Department of Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 5Department of Radiology, University of Cincinnati, Cincinnati, OH, United States, 6Department of Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 7Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Many single-site studies, and increasingly multi-site clinical trials, employ hyperpolarized 129Xe MRI to assess lung structure and function. The lack of reliable imaging 129Xe standards hampers image quantification and rigorous comparisons across research sites. This is particularly true for 129Xe imaging of pulmonary gas-exchange, which must detect the large gaseous resonance and the two, 50-fold-smaller 129Xe dissolved resonances, corresponding to 129Xe dissolved in red bloods cells and adjacent plasma/parenchymal barrier tissues. This work demonstrates the feasibility of constructing practical, thermally-polarized, dissolved-phase 129Xe phantoms that mimic in-vivo resonances and will enable multi-site studies of gas-exchange imaging to be harmonized.
Introduction
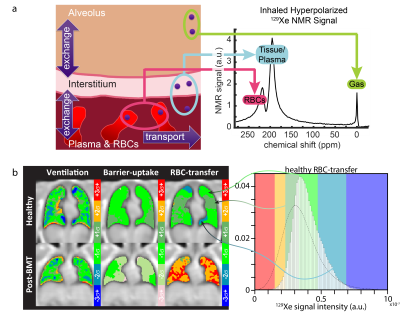
Hyperpolarized 129Xe magnetic resonance imaging (MRI) can quantify lung structure and function in human subjects and is increasingly of interest as an endpoint in multi-site clinical trials. To facilitate protocol harmonization in multi-site trials, standardization, and routine quality control (QC) for ventilation imaging, a thermally polarized, gaseous 129Xe phantom (11.6 bar with T1=580 ms) was created.1 While ventilation is a central component of pulmonary physiology, a key strength of 129Xe is its ability to diffuse from the airspaces of the lung into pulmonary tissues and blood. Dissolved 129Xe exhibits two dissolved-phase resonances—one associated with red blood cells (RBCs) and one associated with non-RBC “barrier tissues”–separated by 19 ppm, Figure 1a. Despite low in-vivo Ostwald solubilities (L, ~0.1-0.2),2 it is possible to directly assess regional gas exchange using approaches such as 3D radial, 1-point Dixon imaging.3-6 Relative to gas-phase ventilation imaging, gas-exchange imaging is more prone to image artifacts (e.g. off-resonance contamination from the 50-fold-larger gaseous signal). Thus, a quantitative reference phantom is much needed to enable rigorous, multi-site trials involving 129Xe gas exchange MRI. This work provides the missing three pieces required to develop a practical, thermally polarized phantom for the two 129Xe dissolved resonances: 1) in-vivo-like spectral properties, 2) high dissolved 129Xe signal, and 3) reduced T1 from >200 s to <1 s. To achieve goals 1 and 2, two high-solubility solvents, dimethyl sulfoxide, with large 129Xe downfield shift (243 ppm, L=0.59) and acetone intermediate shift (171 ppm, L=2.4) were chosen to tune the 129Xe chemical shift (δ). To achieve goal 3, xenon-containing solutions were doped with a highly-paramagnetic, organo-soluble, iron-chelate compound to generate T1s <1 s.Methods
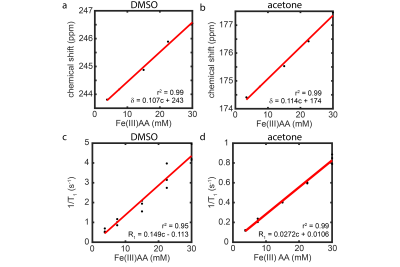
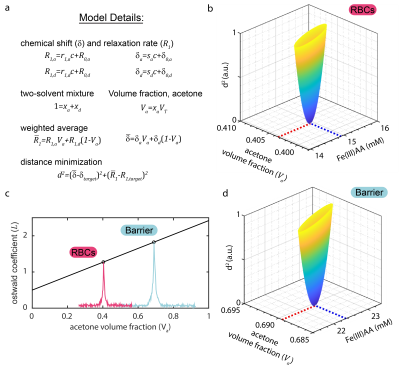
For single solvent phantoms, 35 mM iron(III) acetylacetonate (Fe(III)AA) stock solutions were made and serially diluted to 30, 22.5, 15, 7.5, and 3.8 mM to measure relaxivity (r1), Figure 2. Prior to use, samples were degassed using three freeze/pump/thaw cycles and pressurized with natural abundance xenon (26% 129Xe) at 14.5 psig for 30 min. Phantoms were contained in 60 mL glass pressure tubes (part no. 8648-76, Ace Glass Inc., Vineland, NJ). Mixed solvent phantoms, with desired T1=0.7 s and δRBC=217 ppm and δbarrier =198 ppm, were made based on the model in Figure 3 and pressurized to 75 psig, with agitation for 24 h to ensure equilibrium concentration.Images and spectra were acquired on a 7T Bruker BioSpin (ParaVision 6.0.1, Billerica, MA). T1 times were measured using inversion recovery and fit in MATLAB (Mathworks, Natick, MA). A 1-pt Dixon imaging technique was used to collect a nonselective, 2D-gradient-recalled echo (GRE) image: FOV=80x32 mm, matrix=60x32, BW=100 kHz, TE=0.83 ms, FA=35, TR=200 ms, partial echo=77 %, 1024 averages, and total scan time=1.8 h. Hardware limitations prevented a true TE90 (time needed for π/2 phase separation of the dissolved peaks); therefore, a TE=5π/2 phase separation was implemented.
The δ and T1 of 129Xe resonances depend on Fe(III)AA concentration and solvent type. We measured these relationships (Figure 2) and built a weighted average distance minimization model (Figure 3) to calculate desired acetone volume fraction (Va) and Fe(III)AA concentration needed to reach target values for δ and T1.
Results
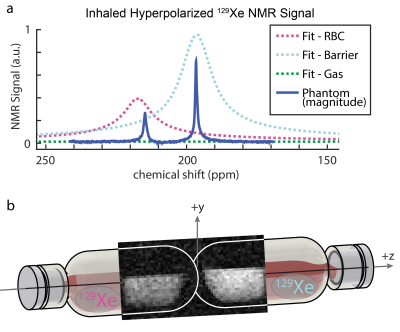
The δDMSO, ~246 ppm, was shifted downfield compared to δacetone, ~176 ppm, in good agreement with literature values,7-9 and the relaxivity (r1) of Fe(III)AA for 129Xe was five-fold higher in DMSO than in acetone. The measured values of T1 and δ (RBC, T1=0.95 s, δRBC=214.8 ppm; and barrier, T1=0.97, δbarrier=196.6 ppm) were in good alignment with predicted values from the model (Figure 3), and the δs aligned well with dissolved 129Xe in-vivo spectroscopy of a subject with healthy lungs, Figure 4a. The 2D-GRE image of the 129Xe dissolved-phase phantoms was acquired in 1.8 h, Figure 4c. The SNR for the RBC and barrier phantoms were 5.1 and 7.8, respectively.Discussion
For this initial work, long scan times were necessary to acquire the GRE image, because moderate pressure, thermally polarized 129Xe at natural isotopic abundance (~26% 129Xe) was used to construct the dissolved-phase phantoms. Moving from natural abundance xenon to isotopically enriched (>85%) 129Xe and increasing the xenon pressure by 30% will decrease scan time 10-fold. Additionally, the use of a center-out pulse sequence (e.g. radial UTE) will permit the acquisition of a true TE90 (0.173 ms at 7T) and reduce signal loss to T2* relaxation. Lastly, acquisition at clinically relevant MRI field strengths for humans (e.g. 3T) will shorten T1 (0.72 ms at 3T), lengthen TE90 (0.52 ms at 3T), allow for an increased flip angle, all of which will increase signal.Conclusion
The practicality of thermally polarized, dissolved-phase 129Xe phantoms is demonstrated using readily available solvents which have high xenon solubility combined with a soluble, paramagnetic, T1 contrast agent. Following improvements outlined in the discussion, expected QC scan time will be <10 min. These phantoms complement the existing 129Xe gas phantom and enable standardization and routine QC for both 129Xe ventilation and gas-exchange imaging in the context of multi-site studies, thus advancing clinical utility 129Xe gas exchange MRI.Acknowledgements
The authors thank the following sources for research funding and support: NIH R01 HL143011 and NIH T32 HL007752.References
1. Bier, E. A. et al. A thermally polarized 129Xe phantom for quality assurance in multi-center hyperpolarized gas MRI studies. Magn Reson Med 82, 1961-1968, doi:10.1002/mrm.27836 (2019).
2. Marshall, H. et al. In vivo methods and applications of xenon-129 magnetic resonance. Prog Nucl Magn Reson Spectrosc 122, 42-62, doi:10.1016/j.pnmrs.2020.11.002 (2021).
3. Wang, Z. et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J 54, doi:10.1183/13993003.00831-2019 (2019).
4. Grist, J. T. et al. Hyperpolarized 129Xe MRI Abnormalities in Dyspneic Patients 3 Months after COVID-19 Pneumonia: Preliminary Results. Radiology 301, E353-E360, doi:10.1148/radiol.2021210033 (2021).
5. Qing, K. et al. Probing Changes in Lung Physiology in COPD Using CT, Perfusion MRI, and Hyperpolarized Xenon-129 MRI. Acad Radiol 26, 326-334, doi:10.1016/j.acra.2018.05.025 (2019).
6. Niedbalski, P. J. et al. Utilizing flip angle/TR equivalence to reduce breath hold duration in hyperpolarized 129Xe 1-point Dixon gas exchange imaging. Magn Reson Med, doi:10.1002/mrm.29040 (2021).
7. Stengle, T. R., Hosseini, S. M., Basiri, H. G. & Williamson, K. L. NMR Chemical Shifts of Xenon in Aqueous Solutions of Amphiphiles: A new Probe of the Hydrophobic Environment. J Solution Chem 13, 779-787 (1984).
8. Segebarth, N., Aїtjeddig, L., Locci, E., Bartik, K. & Luhmer, M. Novel Method for the Measurement of Xenon Gas Solubility Using 129Xe NMR Spectroscopy. J. Phys. Chem. A. 110, 10770-10776 (2006).
9. Solubility Data Series. Krypton, Xenon and Radon - Gas Solubilities 2, (ed H. Lawrence Clever) (Pergamon Press, New York, 1979).
Figures