3509
Backscattering Mueller Matrix polarimetry shows promise for validation of diffusion MRI microstructural features in thick tissue specimens1Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 2Optical Sciences, University of Arizona, Tucson, AZ, United States
Synopsis
Ground truth validation methods are essential to improve the accuracy of biophysical representation by diffusion MRI methods. The objective of this study is to advance PLI methodology for thick tissue imaging of fiber distributions by application of multispectral backscattering polarimetry, and of the Mueller matrix mathematical framework for unmixing contributions from depolarization, diattenutation, and retardance. Our results indicate that orientation features derived from PLI are in concordance with known brain physiology. Due to the consistent presence of retardance even in areas of crossing fibers, the results suggest that retardance angle is most sensitive to microstructural features rather than macroscopic geometry.
Introduction
Ground truth validation methods are essential to improve the accuracy of biophysical representation by diffusion MRI methods. Tractography and orientation distribution metrics in particular can benefit from direct quantitative comparisons to measured features of the tissue environment including compartment volume fractions, g-ratio, fiber orientations and angular distributions of axons and dendrites, but this can be challenging given the microstructural and architectural complexity of brain tissue environments. While radiologic-pathologic correspondence with quantitative histology has enabled formative key observations in this area (1–4) a new class of validation methods has emerged based on the polarized light imaging (PLI) (5). The advantages of PLI validation include stain-free contrast and direct quantitative measurements related to angular features. However, one drawback is that traditional PLI methods are limited to transmission imaging of thin section, limiting utility in vivo and with thick tissue specimens. Furthermore, these methods isolate only the retardance of light, ignoring other relevant polarization effects such as diattenutation and depolarization.The objective of this study is to advance PLI methodology for thick tissue imaging of fiber distributions by application of multispectral backscattering polarimetry, and of the Mueller matrix mathematical framework for unmixing contributions from depolarization, diattenutation, and retardance. Ultimately, advancing these techniques will enable a more robust and widely applicable validation of diffusion MRI data.Methods
Perfusion-fixed and re-hydrated ferret brain specimens were imaged using a Bruker 7T MRI scanner to acquire 3D EPI volumes with 200-micron isotropic resolution and a high angular resolution diffusion imaging (HARDI) encoding scheme with b=10,000 s/mm2 and 87 non-colinear directions as well as an unweighted volume. MRtrix3 software (6,7) was used to perform constrained spherical deconvolution (order=6) and whole brain white matter tractography. Fiber orientation distributions (FODs) and tractograms were visualized in regions corresponding to PLI analysis.After imaging, brains were prepared in thick coronal slabs (>1cm) containing regions with different degrees of white matter fiber complexity: the anterior commissure (AC, single fiber orientation), and multi-tract cerebral white matter (triple fiber orientation). In addition, the full optic chiasm (OC, double fiber orientation) with optic nerve was dissected.
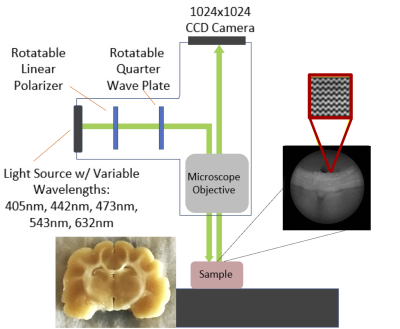
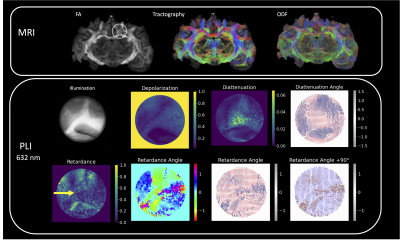
Samples were measured using a Nikon BX41 Mueller Matrix Polarimeter. The instrument operates in reflectance mode (Figure 1) and illuminates the sample sequentially with five visible wavelengths (405 nm, 445 nm, 473 nm, 543 nm, 632 nm) using a fiber-coupled multi-LED light source. Images were collected using a 5X microscope objective and the polarization state of the illumination and detection arms are automatically cycled during acquisition to capture the full polarization interaction of the sample. The acquired data is a spatial mapping of the sixteen Mueller Matrix parameters for the sample.
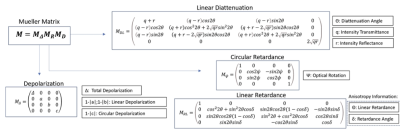
The Mueller Matrix for each pixel was decomposed using the Lu-Chipman Decomposition technique (Figure 2) to isolate effects of depolarization, diattenutation, and retardance using the pySCATMECH package in python 3. Mappings of the resulting polarization parameters were co-registered to dMRI tractography for qualitative comparisons.
Results
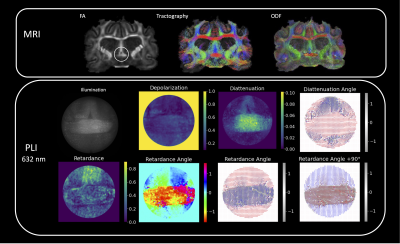
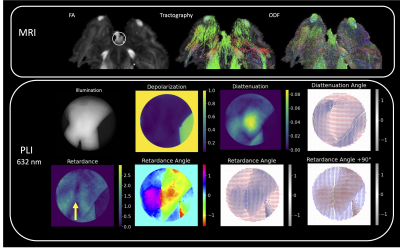
PLI outcomes for regions of different fiber complexity in the ferret brain are shown in figures 3-5 including diffusion FODs and tractograms in the CC, OC and multi-tract WM alongside Mueller matrix metrics derived from PLI at the wavelength of 632 nm. In the AC (Figure 3), we see the clear orientation of the fibers traveling horizontally expressed both in the retardance angle and diattenuation angles, which is consistent with tractography. Furthermore, we observe concordance in the vertically running fibers above the horizontal bundle. In the OC (Figure 4), we also observe that PLI maps the two orientations of the crossing fibers, and remains high in retardance in the area of crossing, suggesting that the retardance and retardance angle are more sensitive to microstructural features, as opposed to macroscopic geometry. In the multi-tract WM (Figure 5), we observe three major sources of retardance angle, which is consistent with the physiological interpretation of three crossing fiber orientations.Discussion
Our results indicate that orientation features derived from PLI are in concordance with known brain physiology. Due to the consistent presence of retardance even in areas of crossing fibers, the results suggest that retardance angle is most sensitive to microstructural features rather than macroscopic geometry. For both the OC and multi-tract WM, we see a deviation between diattenuation and retardance angles, which suggests that these features are not necessarily correlated. This could be due to the decrease in diattenuation in these tissues compared to the AC, resulting in less sensitivity to angular information. Thus, we can conclude that retardance angle may be more appropriate for deriving angular features.Conclusions
Ultimately, these results suggest that backscattering MM-PLI could be a powerful tool for validation of diffusion MRI metrics and orientation features within thick tissue specimens. The MM metric of retardance and the directional information of the retardance angle were shown to have the greatest potential for reporting microscale angular features. Future directions include determination of wavelength dependence of MM metric values and migration of this technique for in-vivo validation.Acknowledgements
We thank Nikon Research Corporation of American for use of the polarimeter system and processing software, as well as Faith Rice and Jennifer Barton for laboratory assistance and instrument maintenance. MRI was performed in the UA translational bioimaging resource (TBIR) and made possible by the NIH small instrumentation grant: S10 OD025016. The authors would like to thank High Performance Computing (HPC) for providing computing resources .References
1. Budde MD, Frank JA. Examining brain microstructure using structure tensor analysis of histological sections. Neuroimage. 2012/07/05. 2012;63(1):1–10.
2. Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014/04/04. 2014;508(7495):207–14.
3. Grussu F, Schneider T, Zhang H, Alexander DC, Wheeler-Kingshott CA. Neurite orientation dispersion and density imaging of the healthy cervical spinal cord in vivo. Neuroimage. 2015/02/06. 2015;111:590–601.
4. Schilling K, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW. Comparison of 3D orientation distribution functions measured with confocal microscopy and diffusion MRI. Neuroimage. 2016/01/26. 2016;129:185–97.
5. Mollink J, Kleinnijenhuis M, Cappellen van Walsum AM van, Sotiropoulos SN, Cottaar M, Mirfin C, et al. Evaluating fibre orientation dispersion in white matter: Comparison of diffusion MRI, histology and polarized light imaging. Neuroimage. 2017 Aug 15;157:561–74.
6. Tournier J, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol. 2012;22(1):53–66.
7. Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation [Internet]. Vol. 202, NeuroImage. Academic Press Inc.; 2019.
Figures