3507
Once upon a chiasm: Reconstruction of mouse optic pathways with ODF-Fingerprinting1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, NYU Langone Health, New York, NY, United States, 2NeuroImaging and Visual Science Laboratory, Departments of Ophthalmology and Radiology, NYU Langone Health, New York, NY, United States, 3Department of Neurosurgery, Perlmutter Cancer Center, Neuroscience Institute, Kimmel Center for Stem Cell Biology, NYU Langone Health, New York, NY, United States
Synopsis
We reconstruct mouse optic pathways with ODF-Fingerprinting in a challenging crossing area of the optic chiasm. Our method replaces the commonly used peak finding approach with pattern matching when reconstructing white matter fiber directions from orientation distribution functions. As reference, we perform analogous reconstructions using Radial Diffusion Spectrum Imaging and Generalized Q-space Imaging. To obtain ground truth information, we inject intravitreally a 0.1M solution of manganese chloride into four C57BL/6N mice. The optic pathways reconstructed with deterministic tractography based on directional information calculated with our approach reach higher agreement with the ground truth than the other two methods.
Introduction
Optic pathways intersect and bend at the optic chiasm, which presents a challenging structure for in-vivo reconstruction techniques [1]. We aim to improve reconstruction quality in this area using ODF-Fingerprinting (ODF-FP) [2] by leveraging its ability to identify white matter (WM) fibers crossing at narrow angles [3].ODF-FP is a lookup technique that reconstructs fiber directions from Orientation Distribution Functions (ODFs) using pattern matching (Figure 1). For any given ODF calculated from a Diffusion Weighted Image (DWI), our method seeks the closest match in a precomputed ODF-dictionary composed of synthetically generated samples. This allows for using more of the information stored in ODFs to reconstruct fiber directions as opposed to the approaches that rely solely on the ODF peak location [4].
In this work, we present an in vivo study of four C57BL/6N mice. The intravitreal injections of manganese chloride solution allow us to obtain contrast augmentation along the optic pathways thus providing ground truth information for our reconstruction based on diffusion MRI (dMRI). Our experiments show that incorporating ODF-FP into dMRI data post-processing produces improved accuracy of the mouse optic pathways virtual dissection with deterministic tractography. Such a precise reconstruction of the optic chiasm suggests that our approach will also be robust for surgical planning and the generation of brain connectivity maps.
Methods
Animals:This study was approved by the Institutional Animal Care and Use Committee (IACUC). All four C57BL/6N mice were females, 7-8 weeks old at the baseline, sacrificed within 24 hours after the follow-up scan.
Data:
We acquired FLASH T1-weighted images (TE/TR=4.5/30ms) with 100x100x100µm resolution and multishell DWIs sampled at 60 directions (distributed on radial lines at b=250,1000,2250,4000s/mm2 interleaved with 5 images at b=0) with 200x200x200µm resolution. Immediately after the baseline scans, we administered intravitreally 1.0µl (Mice 1&2) or 0.5µl (Mice 3&4) of 0.1M solution of MnCl2 to the left (Mice 1&3) or right (Mice 2&4) eye. Then, 24h (Mice 1&2) or 30h (Mice 3&4) later, we repeated the FLASH T1 acquisition.
Image processing:
We registered the baseline and follow-up scans, then calculated the difference images to localize the areas of the optic pathways highlighted by the manganese injection (Figure 2). We denoised our DWIs with the MRtrix3 dwidenoise [5] and calculated ODFs using Radial Diffusion Spectrum Imaging (RDSI) [6] and, for comparison, with Generalized Q-sampling Imaging (GQI) [7]. We then generated a randomized ODF-dictionary [8] of 106 elements containing 0≤N≤3 crossing fibers per voxel, with other parameters matching the dMRI acquisition. We applied ODF-FP [9] to the ODF maps obtained with RDSI to reconstruct fiber directions. Having these, we ran deterministic tractography in DSI Studio [10] using fiber directions computed with each of the three approaches, i.e. GQI, RDSI, and ODF-FP. In all tested cases, we defined 4 regions of interest (ROIs) – two rostral and two caudal – and a seeding region located in the optic chiasm. For tractography, we limited the number of seeds to 1000000, maximum angle to 60°, and Normalized Quantitative Anisotropy (NQA) to 0.03.
Evaluation:
We assessed qualitatively the reconstruction of ipsi- and contralateral tracts that enter the chiasm rostrally and exit it caudally (Figure 3). Then, we quantified the overlapping areas between the reconstructed pathways and the regions highlighted on the manganese-enhanced images (Figure 2). For this, we defined:
$$\mbox{overlap measure} = \frac{volume(\mbox{tractography}\cap\mbox{ground truth})}{volume(\mbox{ground truth})}\cdot100\%$$
Results
The MnCl2 solution gave strong contrast enhancement along the optic nerves and into the chiasm such that we could mark the shape of the optic pathways (Figure 2). However, in Mouse 4 the contrast hardly reached the optic chiasm.The tractography based on fiber directions reconstructed with ODF-FP successfully reproduced the four studied components of the optic pathways, as shown in the representative example of Mouse 2 (Figure 3). On the contrary, the corresponding streamlines obtained with RDSI were visibly thinner, while GQI failed to reconstruct the rostral right – caudal left link (Figure 3). ODF-FP outperformed the other approaches also quantitatively, reaching predominantly higher overlap with the ground truth (Figure 4). Only in Mouse 4, the overlap measures of RDSI and ODF-FP were equal (90%).
Discussion
In dMRI, signals that represent bending and crossing fibers are very much alike if not identical [4]. The fact that both these configurations appear in the optic chiasm makes reconstruction of the optic pathways particularly difficult in vivo [1]. However, ODF-FP overcomes this challenging geometry by treating each ODF as a multidimensional vector and seeking its nearest match in a precomputed ODF-dictionary. This reduces confounding blurring or partial volume effects ubiquitous to ODFs due to spatial and temporal limitations of diffusion sampling schemes [11,12].In this study, we showed for the first time that deterministic tractography executed on top of ODF-FP can reach superior accuracy – with respect to the ground truth information – when reconstructing the optic pathways in vivo in animal model. A limiting factor of our approach is the necessity to generate a dedicated ODF-dictionary, whose parametrization can impact the overall performance of the method [8]. Nonetheless, we believe that ODF-FP adds a significant value to dMRI post-processing pipeline.
Acknowledgements
This project is supported in part by the National Institutes of Health (NIH, R01-EB028774 and R01-NS082436). This work was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, https://www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41-EB017183).References
[1] Deng et al., Applications of manganese-enhanced magnetic resonance imaging in ophthalmology and visual neuroscience, Frontiers in neural circuits, vol. 13, 2019.
[2] Baete et al., Fingerprinting Orientation Distribution Functions in diffusion MRI detects smaller crossing angles, NeuroImage vol. 198, 2019.
[3] Filipiak et al., ODF-Fingerprinting improves reconstruction of fibers crossing at shallow angles: A study on diffusion phantom, ISMRM Annual meeting, 2021.
[4] Jeurissen et al., Diffusion MRI fiber tractography of the brain, NMR in Biomedicine vol. 32, 2019.
[5] Veraart et al., Denoising of diffusion MRI using random matrix theory, NeuroImage vol. 142, 2016.
[6] Baete et al., Radial q‐space sampling for DSI, Magnetic resonance in medicine vol. 76, 2016.
[7] Yeh, Q-space imaging, http://dsi-studio.labsolver.org/course/q-space-imaging-1
[8] Filipiak et al., Two methods to generate an ODF-dictionary for ODF-Fingerprinting, OHBM Annual meeting, 2021.
[9] Baete et al., https://bitbucket.org/sbaete/odffingerprinting/
[10] Yeh, http://dsi-studio.labsolver.org/
[11] Barnett, Theory of Q‐ball imaging redux: Implications for fiber tracking, Magnetic resonance in medicine vol. 62, 2009.
[12] Jensen et al., Resolving power for the diffusion orientation distribution function. Magnetic resonance in medicine vol. 76, 2016.
Figures
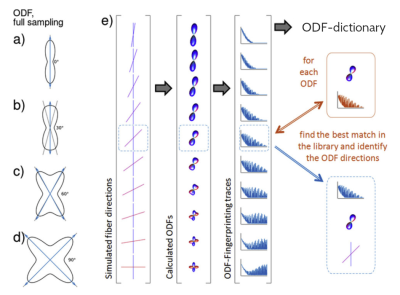
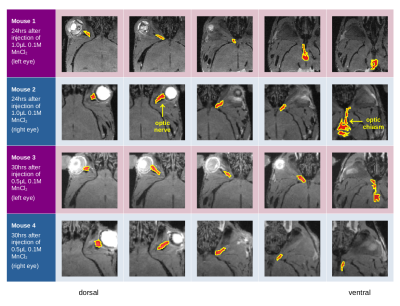
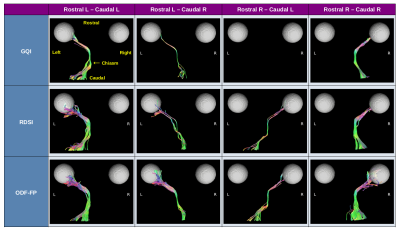
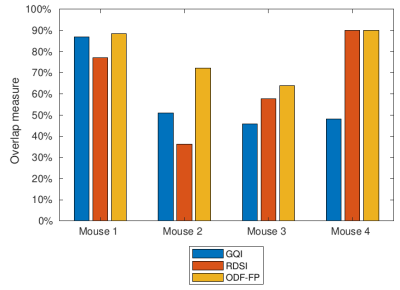
Figure 4. Overlap measure between the optic pathways reconstructed from diffusion-weighted images and the areas highlighted on the manganese-enhanced images.