3505
Flexible FOV image reconstruction using multi-echo 13C imaging with rotating spiral arms1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Electrical and Computer Engineering, UT Dallas, Richardson, TX, United States, 3Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Metabolic imaging with hyperpolarized 13C substrates often detects signals only from small subregions within the prescribed FOV, primarily due to lacking background signals and limited perfusion or metabolic activities in the adjacent tissues. In this study, we propose an imaging method that provides flexible reconstruction options with variable FOV. The proposed pulse sequence was implemented, and the feasibility of the reconstruction method was validated with a 13C phantom.
INTRODUCTION
Spiral imaging, followed by a metabolite-selective RF excitation, provides a fast-imaging option for hyperpolarization (HP) 13C. This imaging approach is particularly suitable for cardiac imaging due to the rapid cardiac motion and coronary blood flow (1, 2). Despite small size of the heart, a large field of view (FOV) is typically prescribed to avoid potential aliasing of neighboring organs such as liver and spleen (3). However, the large FOV requires dense k-space sampling, resulting in unnecessarily long spiral readouts and limited spatial resolution in the reconstructed images. In particular, HP 13C cardiac imaging with a large FOV often only showed the heart region in the reconstructed images, due to lack of metabolic activity in its surrounding tissues. A reduced FOV imaging method was previous proposed by reduced k-space sampling density (4, 5). Additionally, we reported T2* values of HP pyruvate, lactate and bicarbonate in the heart by fitting the signal decay using multi-echo spiral imaging sequence (3). The measured long T2*s were long enough to acquire consecutive multiple echo images using a short spiral readout (e.g., ~5 ms). For instance, T2* of HP [13C]bicarbonate in myocardium was measured as 64.4 ± 2.5 ms. In the current study, we designed a set of short spiral arms, similar to multi-shot spirals, for rotating multi-echo spiral imaging. Variable FOV images can be reconstructed from variable k-space sampling density by combining a selective subset of spirals.METHODS
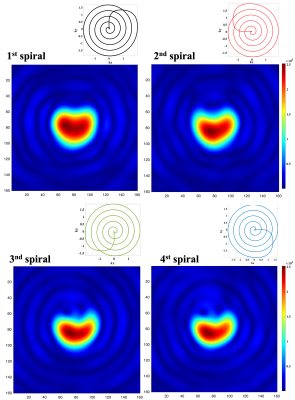
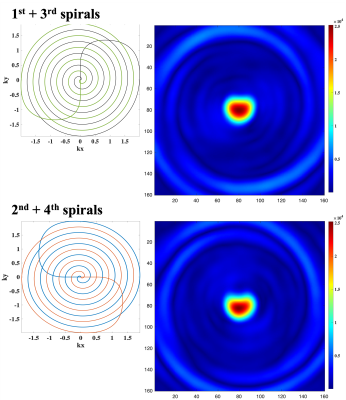
Phantom validationA spectral-spatial RF pulse (3) and the rotating multi-echo spiral imaging trajectories were implemented in a clinical 3T 750w MRI scanner (GE Healthcare, Waukesha, WI). To demonstrate the performance of the proposed sequence, a gadolinium-doped spherical [13C]bicarbonate phantom (1.0 M, diameter = 3 cm) was used with a 13C/1H dual-tuned birdcage transmit/receive RF coil (GE Healthcare). Four spiral readouts were acquired sequentially following the spectral–spatial RF excitation (FOV = 40x40 mm2, spatial resolution = 1x1 mm2, FA = 90°, ST = 25 mm, TE/TR = 21.4/3000 ms, readout length per interleave = 5.35 ms, receiver spectral bandwidth = 186.9858e Hz, 32 averages). The sequence diagram was summarized in figure 1. The acquired data were reconstructed for three FOVs: a) full-scale FOV image reconstruction using four spiral arms, b) half FOV images by combining two facing spiral arms (1st/3rd arms and 2nd/4th arms), and c) quarter FOV images from each spiral arm. The image reconstruction matrix size was set to 160x160.
RESULTS
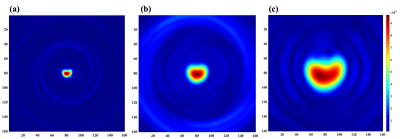
Figure 2 shows the central quarter region of the full-size FOV, reconstructed from each single spiral readout. Each spiral arm was reconstructed separately, and a shirking phantom shape along the number of readouts could be observed. Figure 3 shows reconstructed images with half-size FOV by combining two facing spiral arms. Figure 4 shows the full-size image, reconstructed from the all spiral readouts and also compares the full/half/quarter-sized images. Enlarged phantom images could be reconstructed with increased spatial resolution (voxel size = 2.5x2.5 mm2 in figure 4a, 1.25x1.25 mm2 in figure 4b and 0.625x0.625 mm2 in figure 4c).DISCUSSION and CONCLUSION
The proposed method collects multi-echo images from the rotating spiral arms. Due to the rotating spiral trajectories, the acquired data can be combined, similar to multi-shot image reconstruction. The flexible FOVs reduce potential risks of having aliasing artifacts in the small FOV images by reconstructing the large FOV image. Simultaneously, by comparing multi-echo images, T2* information can be verified. More importantly, the flexible FOV allows us to design the spiral arm to cover a larger k-space area (kmax). Ringing artifacts appeared in all reconstructed images, indicating the truncated k-space coverage. Larger k-space coverage will suppress such artifacts. The phantom results demonstrated that the targeted object in the center of FOV can be zoomed in. Limitations of the proposed approach is the short spiral readout lengths as compared with the T2*. Therefore, heterogenous tissues with short T2* may not qualify for the approach (3). In conclusion, the proposed acquisition and reconstruction method has been demonstrated variable FOV within single acquisition and would further be applied for 13C HP imaging in other small organs such as hearts, prostates, and kidneys.Acknowledgements
National Institutes of Health of the United States (R01 NS107409); The Welch Foundation (I-2009-20190330).References
1. Lau AZ, Chen AP, Ghugre NR, Ramanan V, Lam WW, Connelly KA, et al. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn Reson Med. 2010;64(5):1323-31.
2. Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016;119(11):1177-82.
3. Ma J, Chen J, Reed GD, Hackett EP, Harrison CE, Ratnakar J, et al. Cardiac T2* measurement of hyperpolarized (13) C metabolites using metabolite-selective multi-echo spiral imaging. Magn Reson Med. 2021;86(3):1494-504.
4. Hu X, Parrish T. Reduction of field of view for dynamic imaging. Magn Reson Med. 1994;31(6):691-4.
5. Sedarat H, Kerr AB, Pauly JM, Nishimura DG. Partial-FOV reconstruction in dynamic spiral imaging. Magn Reson Med. 2000;43(3):429-39.
Figures