3501
Fast Quantitative Susceptibility Mapping with Temporal Feature Augmented and Spatiotemporal Sampling Pattern Optimized Network1Cornell University, New York, NY, United States, 2Weill Cornell Medicine, New York, NY, United States
Synopsis
A multi-echo sampling pattern optimization and temporal fusion network for MRI reconstruction is proposed to accelerate data acquisition for quantitative susceptibility mapping (QSM). Experiments show that both features help improve multi-echo image reconstruction. The proposed method was applied to a prospectively under-sampled mGRE acquisition demonstrating better image quality over all comparison methods.
Introduction
Quantitative Susceptibility Mapping (QSM) measures tissue magnetic susceptibility values from the local tissue field data [1] estimated from a multi-echo pulse sequence with large range of echo times to cover both small and large susceptibilities. A recent work [2] accelerated QSM acquisition using 2D incoherent Cartesian under-sampling and unrolled neural network reconstruction. In this work, we optimize both spatiotemporal sampling pattern and reconstruction of mGRE signals in one learning-based approach and show image quality improvement using prospectively undersampled data in healthy subjects.Methods
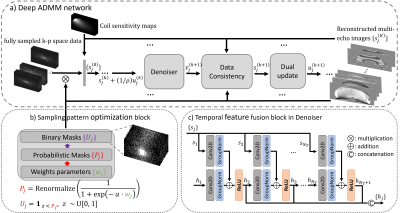
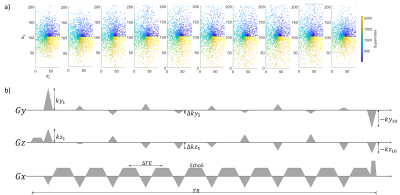
The proposed network architecture is shown in Figure 1. In Figure 1a, a deep ADMM network was used to reconstruct multi-echo complex images simultaneously. This architecture was derived from unrolling an ADMM iterative scheme for image reconstruction and replacing the denoising subproblem with a convolutional neural network. Real and imaginary parts were concatenated into channel dimension. Figure 1b shows the LOUPE-ST [3] component which performs MRI sampling pattern optimization (SPO) extended to the multi-echo case. Figure 1c shows the temporal fusion network that was inserted into the denoiser to capture signal redundancies along echoes.The learned k-space sampling patterns were implemented in a modified GRE sequence for prospective data acquisition. Gradient pulses along the phase and slice encoding directions were added between any two consecutive echoes to allow different phase and slice encodings for each echo within the same TR. To avoid large changes in the phase and slice encoding gradients between two echoes, the following k-space ordering strategy was followed: for each echo , the sampled k-space locations (phase/slice) were first divided into multiple ordered segments of equal size based on their angle with respect to the positive axis. Within each segment, k-space locations were ordered based on their distance with respect to the k-space center. Illustration of the proposed segmented k-space ordering and pulse sequence design is shown in Figure 2.
Cartesian fully sampled k-space data were acquired in 13 healthy subjects using a 3D mGRE sequence on a 3T GE scanner with a 32-channel head coil. Imaging parameters included FA = 15°, FOV = 25.6 cm, TE1 = 1.972 ms, TR = 36 ms, #TE = 10, ΔTE = 3.384 ms, acquisition matrix = 256×206×80 (readout × phase encoding × slice encoding), voxel size = 1×1×2 mm3, BW = 64 kHz. Total scan time was 9:30 min per subject. Coil sensitivity maps of each echo were estimated with ESPIRiT [4]. The central 200 locations along the readout direction were extracted and 8/1/4 subjects (1600/200/800 slices) were used as training, validation, and test datasets, respectively.
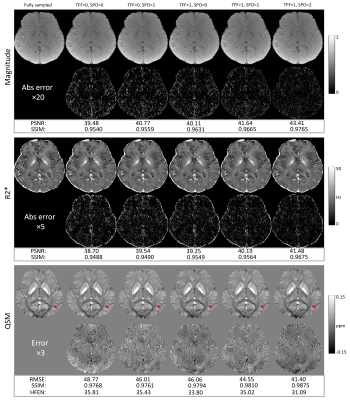
An ablation study was performed on the retrospectively under-sampled data comparing the change in image quality metrics induced by the multi-echo sampling pattern optimization and temporal fusion network separately and together. The temporal fusion network were either absent (TFF=0) or present (TFF=1). The SPO had three variations: no sampling pattern optimization (SPO=0), single echo optimization (SPO=1, same pattern across echoes) and multiple echo optimization (SPO=2, pattern varies across echoes).
The proposed method was compared with two previously proposed accelerated MRI reconstruction methods: LLR [5] and MoDL [6].
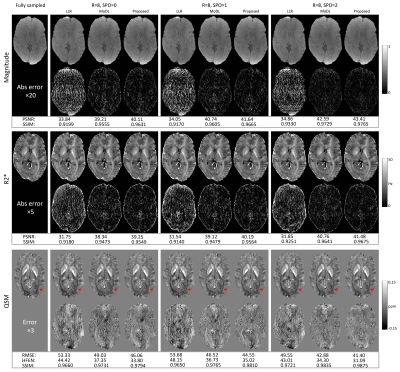
For a retrospective study, an acceleration factor (12.5% under-sampling ratio) was applied on the fully sampled acquired k-space dataset. For a prospective study, Cartesian under-sampled k-space data was prospectively acquired in four test subjects using the modified 3D mGRE sequence with the same 3T GE scanner and imaging parameters. Different sampling patterns with were applied during prospective scans and compared. Corresponding scan time was 1:20 mins. For reference, the subject underwent a second scan using the default imaging protocol. This protocol had identical imaging parameters except for R=2 uniform under-sampling reconstruction using the SENSE implementation on the scanner. Corresponding scan time was 3:44 mins.
Results
Figure 3 shows the results from the ablation study. Reconstruction errors were progressively reduced in all maps multi-echo sampling pattern optimization and recurrent temporal network components were added. White matter tracts (red solid arrows) in QSMs were blurry for all reconstructions except for the optimized k-space pattern with TFF (TFF=1, SPO=2).Figure 4 shows a comparison of the proposed method with LRR and MoDL. LLR show more reconstruction artifacts and larger errors compared to MoDL and the proposed method. No visual artifacts were seen in either MoDL or the proposed method. Moreover, the proposed deep ADMM with TFF had a slightly lower error compared to MoDL. Additionally, white matter tracts (red solid arrows) were blurry in all QSM reconstructions except for the optimized k-space pattern with TFF (TFF=1, SPO=2).
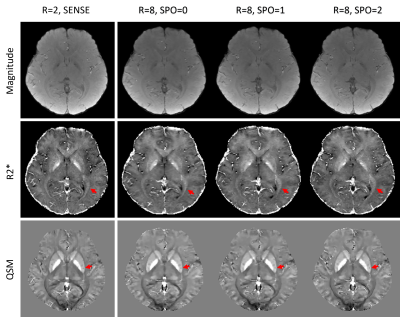
The results for the prospectively under-sampled scans () are shown in Figure 5. Using the default SENSE acquisition with as reference, depictions of white matter tracts in R2* maps (red solid arrows in the 2nd row) progressively improved from SPO=0, 1 to 2. The depiction of calcium deposits inside the putamen on QSM (red solid arrows in the 3rd row) were unsharp in SPO=0 and 1 but were sharp in SPO=2.
Discussion and conclusions
We proposed a method to optimize mGRE signal acquisition and image reconstruction for accelerating QSM. Experimental results showed effectiveness of both SPO and TFF modules. Prospective experiment using the optimized multi-echo sampling pattern shows the practical feasibility of the proposed method.Acknowledgements
No acknowledgement found.References
1. de Rochefort, Ludovic, et al. Magnetic Resonance in Medicine 63.1 (2010): 194-206.
2. Gao, Yang, et al. NeuroImage 240 (2021): 118404.
3. Zhang, Jinwei, et al. MLMIR. Springer, Cham, 2020.
4. Uecker, Martin, et al. Magnetic resonance in medicine 71.3 (2014): 990-1001.
5. Zhang, Tao, et al. Magnetic resonance in medicine 73.2 (2015): 655-661.
6. Aggarwal, Hemant K, et al. IEEE TMI 38.2 (2018): 394-405.
Figures