3499
Accelerated 5D Echo Planar J-Resolved Spectroscopic Imaging and Dictionary Learning Reconstruction: Pilot Findings in Obstructive Sleep Apnea1Radiological Sciences, University of California-Los Angeles, Los Angeles, CA, United States, 2School of Nursing and Brain Research Institute, University of California-Los Angeles, Los Angeles, CA, United States
Synopsis
Obstructive sleep apnea (OSA) affects 10% of the population, and is associated with brain injury. Neurochemical changes in the brain of OSA patients can be recorded using 5D echo-planar J-resolved spectroscopic imaging. Accelerated acquisition is achieved with non-uniform undersampling, which requires data reconstruction that can be done with compressed sensing (CS). We implemented CS with a hybrid DLTV reconstruction method combining dictionary learning (DL) and total variation (TV), and compared its performance with Perona-Malik (PM) reconstruction. The metabolite ratios were consistent with both DLTV and PM while DLTV recovered the metabolite peaks near residual water better in many voxels.
Introduction
Obstructive sleep apnea (OSA) is a common multisystem sleep disorder associated with obesity and age, factors likely underlying the increasing prevalent of the sleep disorder (1-3). OSA is associated with disrupted sleep, cardiovascular and cognitive functions likely linked to brain injury (4-7). Five-dimensional (5D) echo-planar J-resolved spectroscopic imaging (EP-JRESI) scans demonstrated several neurochemical changes in multiple brain regions of OSA compared to healthy controls (HC) (8-10). Due to the increased scan time required for full encoding along two spatial and the 2nd spectral dimensions, acceleration by non-uniform undersampling (NUS) and compressed sensing reconstruction (CS) is commonly employed. In this study, we have evaluated the performance of a hybrid Dictionary Learning (DL)-Total Variation (TV) (DLTV) reconstruction of the NUS 5D EP-JRESI data acquired in OSA patients and HC, and compared it with Perona-Malik (PM) reconstruction (11-14).Materials and Methods
We scanned eight OSA (age:38.6±8.9years) and eight HC (age:26.5±7.3years) using a 3T Siemens MRI scanner with a 32-channel head receive coil. A maximum echo-based 5D EP-JRESI sequence using two-pairs of adiabatic full passage RF pulses was used (8-10,15). The acquisition parameters were: TR/TE/Avg=1200ms/34ms/1, 32kx,16ky,8kz,512t2,64t1, field of view=24x24x12cm3 resulting in extractable voxel resolution of 1.5x1.5x1.5cm3. Spectral bandwidths along t2 and t1 were 1190Hz and ±250Hz. An 8x NUS was imposed along t1, ky and kz. WET-suppression was used for the global suppression of water (16). This was followed by a non-water suppressed scan with only the first t1 increment.PM used gradient sparsity while DLTV used a combination of gradient sparsity and DL based sparsity for reconstruction (11,14). DLTV learned dictionary from TV filtered data in each iteration using K-SVD algorithm (17). Reconstruction was accelerated by operating DLTV in a custom-3D k-space formed by stacking the direct spectral dimension (F2), so that we train a single dictionary for F2, instead of separate dictionaries for each F2 point. The associated cost function minimization was $$\min_{D,m_f,\rho_\mathscr{R},\rho_\mathscr{I}}\sum_i(\parallel\rho_{\mathscr{R},i}\parallel_0+\parallel\rho_{\mathscr{I},i}\parallel_0)+\mu\mid\triangledown m_f\mid_1+ \nu\parallel{F_um_f-s_f}\parallel_2^2 s.t \left\{\begin{array}{cc} \parallel{D\rho_{\mathscr{R},i} -\mathbb{R}_i\mathscr{R}(m_f)}\parallel_2^2<\epsilon\\ \parallel{D\rho_{\mathscr{I},i} -\mathbb{R}_i\mathscr{I}(m_f)}\parallel_2^2<\epsilon\\ \end{array}\right\} \forall i$$ where, $$$s_f$$$ and $$$m_f$$$ are the acquired and reconstructed custom-3D k-space. $$$F_u$$$ computes forward and inverse Fourier transforms in image and temporal domains, and set the values at unacquired locations of k-space as zeros. $$$\mu$$$ and $$$\nu$$$ controls gradient sparsity and data consistency. $$$D$$$ is a real valued, adaptively learnt dictionary. $$$\mathbb{R}$$$ extracts 3D blocks from the custom-3D space and $$$i$$$ is the block number. $$$\rho$$$ is the sparse representation of block. $$$\mathscr{R}$$$ and $$$\mathscr{I}$$$ denotes the real and imaginary components.
Results
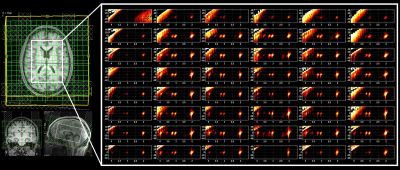
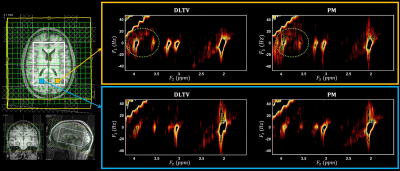
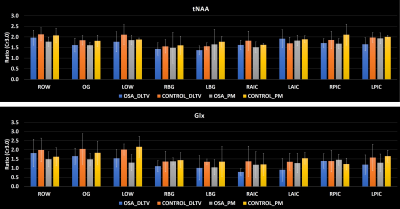
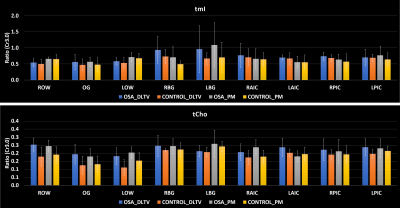
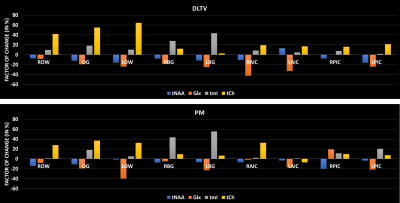
Fig. 1 shows the localization volume on a T1-weighted axial MRI of a 5D EPJRESI data acquired from 36-year-old OSA patient and the reconstructed multi-voxel spectra from a selected slice. While the dominant water tail is prevalent in the frontal region, occipital region shows better reproduction of the metabolites. Fig. 2 shows extracted voxels (3.4ml volume) from occipital gray (OG) and left occipital white (LOW) reconstructed using PM and DLTV. DLTV showed less influence of water tail on the nearby creatine (Cr) 3.9 peak compared to PM in the LOW region. Fig. 3 and 4 show selected metabolite ratios (with respect to Cr 3.0) after the PM and DLTV reconstructions in the following regions: OG, LOW, right occipital white (LOW), right basal ganglia (RBG), left basal ganglia (LBG), right anterior insular cortex (RAIC), right posterior insular cortex (RPIC), left anterior insular cortex (LAIC) and left posterior insular cortex (LPIC). Fig. 5 shows the alterations of metabolite ratios in OSA in Figs. 2-3. Both DLTV and PM showed the same trend of metabolite changes in OSA compared to healthy controls in all regions except at LAIC, where tNAA(NAA+N-acetyl-aspartyl-glutamate)/Cr and tCho(Choline+glyceryl-phosphocholine+phosphocholine)/Cr were increased by 30% and 17% respectively in DLTV, whereas it was decreased by 3% and 7% in PM.Discussion
Both PM and DLTV were able to satisfactorily reconstruct the 8x NUS 5D EP-JRESI data of OSA and HC. While DLTV showed better robustness in recovering metabolites closer to the water and fat peaks, the difference did not drastically change the outcome of quantitation. The improvement with DLTV can be understood based on the fact that the TV-filtered training data helps DL to find sparser representation, leading to an overall improved performance. The frontal gray and white matter in some of the datasets were not quantifiable due to the less effective outer volume suppression near sinus regions. Voxels with inadequate spectral quality were excluded from the analysis. OSA patients showed reduced tNAA/Cr and Glx(glutamine+glutamate)/Cr, and increased tmI(myo-inositol+glycine)/Cr and tCho/Cr compared to HC in the insular cortex, basal ganglia and occipital regions (18-19). The increase in tmI/Cr was the largest in basal ganglia with both DLTV and PM (20). Reduced tNAA/Cr ratio can be a result of impaired neuronal viability and/or integrity (21).Conclusion
It is observed that a combination of DL and gradient sparsity can improve the CS reconstruction of metabolite peaks in proximity to residual water and fat compared to the CS reconstruction using only gradient sparsity. The trend in impairment of relative metabolite concentrations was found to be consistent with both PM and DLTV in basal ganglia and occipital regions. Studies with a larger cohort of longitudinal subjects is required to further validate the metabolite changes observed in the current study.Acknowledgements
Supported by the National Heart, Lung and Blood Institute HL135562.References
1. Malhotra A, White DP. Obstructive sleep apnoea. Lancet 2002;360:237–45.
2. Li Y1, Veasey SC. Neurobiology and neuropathophysiology of obstructive sleep apnea. Neuromolecular Med. 2012;14(3):168-79.
3. Sarchielli P, Presciutti O, Alberti A,et al. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15(10):1058-64.
4. Wong JL, Martinez F, Aguila AP, et al. Stress in obstructive sleep apnea. Scientific Reports. 2021 Jun 16;11(1):1-9.
5. Park B, Palomares JA, Woo MA, Kang DW, et al. Disrupted functional brain network organization in patients with obstructive sleep apnea. Brain and behavior. 2016 Mar;6(3):e00441.
6. Macey PM. Damage to the hippocampus in obstructive sleep apnea: a link no longer missing. Sleep. 2019 Jan 1;42(1):zsy266.
7. Macey PM, Kheirandish-Gozal L, Prasad JP, et al. Altered regional brain cortical thickness in pediatric obstructive sleep apnea. Frontiers in neurology. 2018 Jan 22;9:4.
8. Wilson NE, Iqbal Z, Burns BL, et al. Accelerated five-dimensional echo planar J-resolved spectroscopic imaging: Implementation and pilot validation in human brain. Magn Reson Med. 2015
9. Sarma MK, Macey PM, Iqbal Z, et al. Accelerated Five-Dimensional Echo Planer J-resolved spectroscopic imaging: Pilot findings in untreated and CPAP treated Obstructive Sleep Apnea. ISMRM‐ESMRMB Annual Meeting 2016; Singapore. 2016.
10. Sarma MK, Macey PM, Saucedo A, et al. Cerebral Metabolite Changes and Sleep Correlates in Obstructive Sleep Apnea. ISMRM‐ESMRMB Annual Meeting 2018; Paris, France. 2018.
11. Joy A, Paul JS. Multichannel compressed sensing MR image reconstruction using statistically optimized nonlinear diffusion. Magn Reson Med 2017;78(2):754-762.
12. Joy A, Jacob M and Paul, JS. Compressed sensing MRI using an interpolation‐ free nonlinear diffusion model. Magn Reson Med 2021;85(3):1681-1696.
13. Ravishankar S, Bresler Y. MR image reconstruction from highly undersampled k-space data by dictionary learning. IEEE transactions on medical imaging. 2010;30(5):1028-41.
14. Rubinstein R, Bruckstein AM, Elad M. Dictionaries for sparse representation modeling. Proceedings of the IEEE. 2010;98(6):1045-57.
15. Garwood M and DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 2001;153(2):155-177.
16. Ogg RJ, Kingsley RB and Taylor JS. WET, a T1-and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. JMagn Reson, Series B, 1994;104(1):1-10.
17. Aharon M, Elad M, Bruckstein A. K-SVD: An algorithm for designing overcomplete dictionaries for sparse representation. IEEE Trans Sig Processing. 2006;54(11):4311-22.
18. Macey PM, Sarma MK, Prasad JP, et al. Obstructive sleep apnea is associated with altered midbrain chemical concentrations. Neuroscience. 2017 Nov 5;363:76-86.
19. Xia Y, Fu Y, Xu H, et al. Changes in cerebral metabolites in obstructive sleep apnea: a systemic review and meta-analysis. Sci Rep. 2016 Jun 28;6:28712.
20. Robertson NJ, Lewis RH, Cowan FM, et al. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res. 2001 Dec;50(6):692-700.
21. Kamba M, Inoue Y, Higami S, et al. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry 2001;71:334–39.
Figures