3488
SuperRes-EPTI: in-vivo mesoscale distortion-free dMRI at 500μm-isotropic resolution using short-TE EPTI with rotating-view super resolution1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
To achieve high spatial resolution in-vivo dMRI (mesoscale at isotropic 500μm), we propose a novel SuperRes-EPTI technique that combines our recently developed EPTI technique with a rotating-view super-resolution acquisition and reconstruction. The SuperRes-EPTI can achieve an overall 3.5× SNR gain (SuperRes×EPTI = 2.7×1.3), while providing images completely-free from distortions that allow for improved super-resolution reconstruction and high effective resolution. The SuperRes acquisition scheme was designed to avoid spin-history artifacts, and incorporates rigid motion correction between thick-slice volumes for high motion-robustness. In-vivo distortion-free dMRI data were acquired at 500μm-isotropic resolution at 3T that reveals detailed fibers in gray matter.
Introduction
High-resolution in-vivo diffusion MRI (dMRI) is important to resolve brain’s detailed structures1,2, but is technically challenging due to the inherent lower SNR of smaller voxels, severe distortion and blurring as well as motion sensitivity. To improve SNR efficiency, several emerging methods have been developed through slab encoding, including simultaneous multi-slab imaging3-5 and SMS-gSlider1,6, which are often combined with parallel imaging to mitigate distortions and have shown promising results for submillimeter dMRI7. These techniques could still suffer from several limitations: distortion and blurring, spin-history striping artifacts, the need for navigator acquisition or high SAR-deposition from specialized RF pulses. Another promising approach to increase SNR efficiency is super-resolution acquisition and reconstruction8-10, which acquires multiple thick-slice volumes with different orientations to resolve the high-resolution volume, which can avoid extra SAR-deposition and spin-history striping artifacts. However, motion and dynamic distortion (caused by motion and eddy currents) between volumes will compromise the fidelity and resolution of the super-resolution approach, limiting its ability to achieve ultra-high resolution.In this work, to address these issues and achieve in-vivo mesoscale dMRI. we propose a SuperRes-EPTI technique that combines our recently developed Echo-Planar Time-resolving Imaging (EPTI)11,12 technique with a rotating-view super-resolution acquisition9. The SuperRes-EPTI can achieve an overall 3.5× SNR gain (SuperRes×EPTI = 2.7×1.3), while providing images completely-free from distortions that enable improved super-resolution reconstruction and high effective resolution.
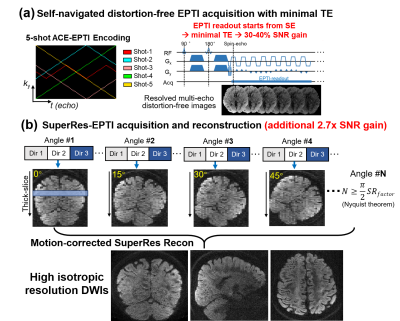
In SuperRes-EPTI, thick-slice volumes of different slice angles will be acquired using a recently developed accelerated echo-train shifted EPTI (ACE-EPTI)12 acquisition to achieve minimal TE (39ms), providing a 30-40% SNR gain over conventional EPI. It provides multi-echo images free from distortion and blurring by resolving signal evolutions across the readout. Eliminating spatial blurring along phase-encoding (PE) can help preserve spatial resolution (there can be >30% of blurring in conventional EPI at 500μm resolution even using in-plane acceleration of R=4). Eliminating the distortion can not only preserve spatial resolution, but also improve super-resolution reconstruction by having consistent geometry across thick-slab volumes when there is subject motion or B0 field change. The thick-slice volumes acquired at different angles will then be used to reconstruct the high-resolution volume8-10. The SuperRes acquisition scheme in this work was designed to avoid spin-history artifacts, and inter-shot physiological noise removal and motion correction between thick-slice volumes were incorporated into the proposed SuperRes-reconstruction framework for high motion robustness. Preliminary dMRI data were acquired at 500μm isotropic resolution in-vivo using SuperRes-EPTI at 3T with high image quality that reveals detailed fibers in gray matter.
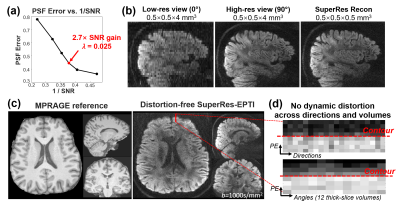
Methods
SuperRes-EPTI acquisition: Each thick-slice volume is first acquired using a 5-shot ACE-EPTI acquisition to achieve distortion-free imaging at 500μm with self-navigated physiological motion correction (Fig. 1a, using the center k-space). The echo-train shifted readout starts at spin-echo with minimized TE (TESE = 39 ms at 500μm, b=1000s/mm2), which has been demonstrated to provide ~30% SNR gain over EPI at 0.86-mm resolution12. A phase-corrected subspace reconstruction12-14 is employed to recover multi-echo images across EPTI readout. Then, thick-slice volumes will be acquired at different slice orientations (rotated around the anterior-posterior axis, PE). Fig.1b illustrates the designed SuperRes acquisition framework. It acquires different diffusion directions first for each slice orientation to avoid spin-history artifacts. To recover high-resolution volume for a given super-resolution factor (SRfactor), N low-resolution rotating volumes, N≥π/2×SRfactor, should be acquired based on the Nyquist-sampling theorem. Therefore, we acquired N=12 angles for a SRfactor of 8 for 500μm-iso dMRI, with a 15° increment, that can offer 2.7× SNR gain (Fig.2a).A motion-corrected SupreRes reconstruction is proposed for EPTI data to recover high-resolution volumes: $$min\parallel AMI_{HR}-I_{LR}\parallel_2^2+\lambda\parallel I_{HR}\parallel_2^2$$
where ILR are the low-resolution volumes, IHR is the target high-resolution volume, M is rigid-motion transforms, A is encoding matrix constructed based on 90°-180° slice profiles, λ controls the Tikhonov regularization.
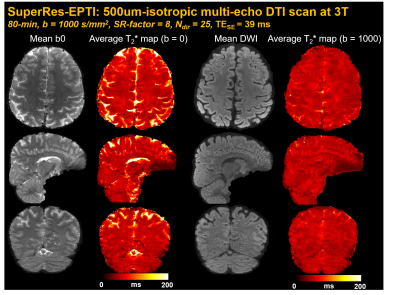
500μm-isotropic in-vivo dMRI: 25-direction diffusion data were acquired using SuperRes-EPTI at 3T Siemens Prisma with a 32-channel coil. Imaging parameters: SRfactor=8, N=12, Nshot=5, SMS=2, b-value=1000s/mm2, echo-spacing=1.54ms, TErange=39-136ms (64-echoes), TR=3.1s, acquisition time ~80 minutes.
Results
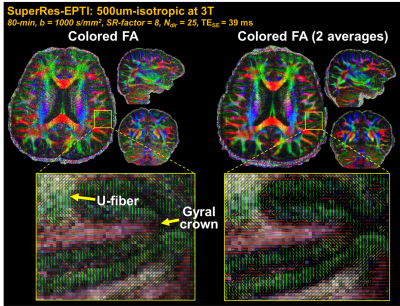
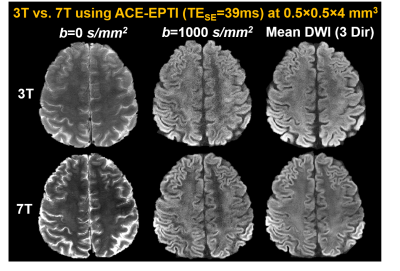
λ was chosen based on the PSFerror vs. 1/SNR L-curve as shown in Fig.2a, for a SNR gain of 2.7× with moderate errors (corresponding to ~14% side-lobe). Fig.2b shows that the reconstructed SuperRes-EPTI image recovers the high spatial resolution at 500μm-isotropic resolution, similar to the high in-plane resolution view of the thick-slice images. The distortion-free high-resolution diffusion-weighted images (DWIs) show the same geometry as the MPRAGE reference (Fig.2c), and no eddy-current/motion-induced dynamic distortion was observed across low-resolution volumes or diffusion directions (Fig.2d), demonstrating the distortion-free capability of SuperRes-EPTI.Fig.3 shows the high image quality of the mean non-DWI and DWI data together with the averaged fitted T2* maps at 500μm-iso. The corresponding colored-FA maps and tensor results are shown in Fig.4. Although SNR is still limited at 3T, high-quality tensors were obtained and revealed clear U-fibers and fibers in the gyral crowns and walls. The short-TE acquisition also enables high SNR imaging at 7T, where T2 values are much shorter. Fig.5 shows the preliminary results acquired by ACE-EPTI at 7T with higher SNR than 3T.
Conclusion
SuperRes-EPTI provides significant improvement of SNR efficiency for distortion-free in-vivo dMRI at high isotropic spatial resolution (500μm). Future work will investigate the application of SuperRes-EPTI at 7T for higher SNR.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB019437, P41EB030006) and the instrumentation Grants (S10OD023637, S10-RR023401, S10-RR023043, and S10-RR019307).
References
1 Setsompop, K. et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magnetic resonance in medicine 79, 141-151, doi:10.1002/mrm.26653 (2018).
2 Schilling, K. et al. Confirmation of a gyral bias in diffusion MRI fiber tractography. Human brain mapping 39, 1449-1466, doi:10.1002/hbm.23936 (2018).
3 Frost, R., Miller, K. L., Tijssen, R. H., Porter, D. A. & Jezzard, P. 3D multi-slab diffusion-weighted readout-segmented EPI with real-time cardiac-reordered K-space acquisition. Magnetic resonance in medicine 72, 1565-1579, doi:10.1002/mrm.25062 (2014).
4 Holtrop, J. L. & Sutton, B. P. High spatial resolution diffusion weighted imaging on clinical 3 T MRI scanners using multislab spiral acquisitions. J Med Imaging (Bellingham) 3, 023501, doi:10.1117/1.JMI.3.2.023501 (2016).
5 Engstrom, M. & Skare, S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magnetic resonance in medicine 70, 1507-1514, doi:10.1002/mrm.24594 (2013).
6 Wang, F. et al. Motion-robust sub-millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magnetic resonance in medicine 80, 1891-1906, doi:10.1002/mrm.27196 (2018).
7 Wang, F. et al. In vivo human whole-brain Connectom diffusion MRI dataset at 760 microm isotropic resolution. Sci Data 8, 122, doi:10.1038/s41597-021-00904-z (2021).
8 Plenge, E. et al. Super-resolution methods in MRI: can they improve the trade-off between resolution, signal-to-noise ratio, and acquisition time? Magnetic resonance in medicine 68, 1983-1993, doi:10.1002/mrm.24187 (2012).
9 Shilling, R. Z. et al. A super-resolution framework for 3-D high-resolution and high-contrast imaging using 2-D multislice MRI. IEEE Trans Med Imaging 28, 633-644, doi:10.1109/TMI.2008.2007348 (2009).
10 Vis, G., Nilsson, M., Westin, C. F. & Szczepankiewicz, F. Accuracy and precision in super-resolution MRI: Enabling spherical tensor diffusion encoding at ultra-high b-values and high resolution. NeuroImage 245, 118673, doi:10.1016/j.neuroimage.2021.118673 (2021).
11 Wang, F. et al. Echo planar time-resolved imaging (EPTI). Magnetic resonance in medicine 81, 3599-3615, doi:10.1002/mrm.27673 (2019).
12 Dong, Z., Wang, F., Wald, L. & Setsompop, K. SNR-efficient distortion-free diffusion relaxometry imaging using ACcelerated Echo-train shifted EPTI (ACE-EPTI). bioRxiv (2021).
13 Dong, Z., Wang, F., Reese, T. G., Bilgic, B. & Setsompop, K. Echo planar time-resolved imaging with subspace reconstruction and optimized spatiotemporal encoding. Magnetic resonance in medicine 84, 2442-2455, doi:10.1002/mrm.28295 (2020).
14 Liang, Z.-P. in 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 988-991 (IEEE).
Figures