3474
Dual-domain Self-supervised Learning for Accelerated MRI Reconstruction1Yale University, New Haven, CT, United States, 2Hyperfine Research, Guilford, CT, United States, 3New York University, New York, NY, United States
Synopsis
We present a self-supervised approach for accelerated non-uniform MRI reconstruction, which leverages self-supervision in k-space and image domains. We evaluated the performance on both simulation and real data, where fully sampled data is unavailable. The experimental results on a non-uniform MRI dataset demonstrate that the proposed method can generate reconstruction that approaches the accuracy of the fully supervised reconstruction. Furthermore, we show that the approach can be applied to highly challenging real-world clinical MRI reconstruction acquired on a low-field (64 mT) MRI scanner with no data available for supervised training while demonstrating improved perceptual quality as compared to traditional reconstruction.
Introduction
Prolonged MR imaging sessions are impractical as they lead to increased patient discomfort and increased accumulation of motion artifacts and system imperfections in the image. As such, there is significant interest in accelerating MRI while maintaining high image fidelity. Numerous efforts have been made for reconstructing high-quality images with undersampled data using deep learning (DL). However, most DL-based reconstruction methods are limited to uniform patterns and are supervised, thus requiring paired fully-sampled acquisitions for ground-truth1,2,3,4. These requirements are impractical as several real-world MRI use-cases may not have the time/resources to fully sample k-space for supervised training or may prefer non-uniform sampling for its motion robustness advantages, among others. For example, low-field portable MRI may exploit accelerated non-uniform k-space acquisition for increased robustness. In this work, we present Dual-domain Self-supervised (DDSS) reconstruction, a self-supervised learning method for accelerated non-uniform MRI reconstruction.Materials and Methods
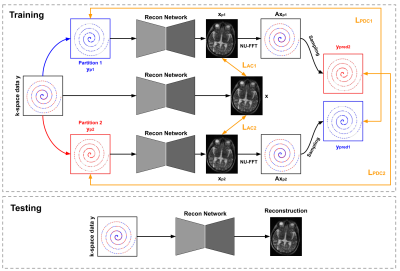
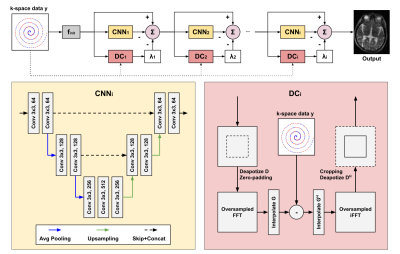
Data Preparation: We used both simulated and real non-uniform data. For the simulation studies, we randomly select 505 T1-weighted and 125 T2-weighted MRI from the Human Connectome Project (HCP)5. To generate non-uniformly undersampled data, we use a variable density sampling pattern where the sampling density decays from the k-space center at a quadratic rate6. We generate two sampling trajectory settings with acceleration factors of 2 and 4. 476 T1-weighted and 104 T2-weighted images are used for training and 29 T1-weighted and 21 T2-weighted MR images for evaluation. For the clinical MRI studies, we collect 106 FLAIR and 112 FSE-T2w 3D brain MR images acquired using a low-field portable MR system (Hyperfine, SwoopTM). Both FLAIR and FSE-T2w images were acquired using a variable density sampling pattern with an acceleration factor of 2. All the clinical data is used in training, and qualitative evaluation is performed on separated patient data with pathological findings. Framework andNetwork Architecture: The DDSS is trained only on non-uniform undersampled data with self-supervision coming from both k-space and image domains. The DDSS framework is illustrated in Fig 1. The undersampled k-space data are first partitioned into two disjoint sets. For k-space self-supervision, each disjoint set is fed into the same reconstruction network in parallel, where the other set is used for supervision in k-space. For image domain self-supervision, image appearance between the reconstructions of the partitioned data and the reconstruction of the original undersampled data are encouraged to be consistent. L1 losses are computed for both k-space self-supervision and image domain self-supervision, and were minimized using Adam optimizer with a learning rate 3x10-5. We use a variational network7 based on the unrolling of a traditional iterative nonlinear reconstruction as our reconstruction network (Fig 2).
Evaluation: As an upper bound, we compare the DDSS against a supervised strategy where the same reconstruction model was trained in a fully supervised fashion with ground truth available. To further evaluate the advantages of dual-domain training, we compare against a k-space domain self-supervised (KDSS) model where only the k-space domain self-supervision is used for reconstruction model training. For quantification on the simulated data, we measure SSIM, PSNR, and MSE. For real data study, all real non-uniform data is used for self-supervised training and is qualitatively evaluated as there is no ground truth reconstruction for quantification.
Results
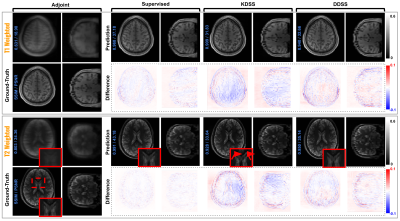
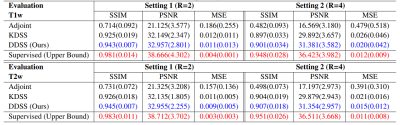
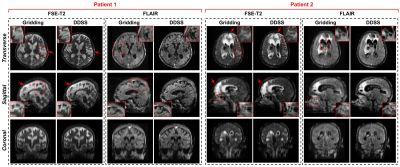
For the simulation studies, quantitative results are summarized in Fig 4. As we can observe, with acceleration R = 2 on T1w reconstruction, DDSS can achieve SSIM = 0.943 which is notably higher than the KDSS with SSIM = 0.925, thus narrowing the gap in performance to the fully supervised upper bound. Similar observations can be found for T2w reconstruction experiments where the DDSS outperforms the KDSS by achieving the SSIM of 0.945 over the KDSS with 0.926. While the overall performance decreases for all methods as the acceleration factor increases from R = 2 to R = 4, DDSS still outperforms KDSS on both T1w and T2w reconstruction tasks in terms of PSNR, SSIM and MSE. Qualitative comparisons are visualized in Fig 3. Even though the supervised model using fully sampled ground truth during training achieves the best quantitative results, the reconstructions from the supervised model and the DDSS are comparable qualitatively. For the clinical studies, we compare it against the default gridding reconstruction from the low-field (64mT) portable MR system. Qualitative results are visualized in Fig 5, where we visualize results from FSE-T2w and FLAIR scans of two stroke patients. Notably, the gridding reconstructions suffer from blurring due to the accelerated data acquisition protocols, while the self-supervised DDSS reconstructions produce much sharper image quality leading to enhanced visualization of neuroanatomy.Discussion and Conclusions
The proposed method offered promising solutions to two major difficulties in MRI reconstruction. First, the proposed self-supervised method allows the MRI reconstruction network to be trained without using any fully sampled MRI data Second, our method is generalized for non-uniform MRI reconstruction, making the method readily applicable to advanced MR data acquisition techniques. We demonstrated the effectiveness of the proposed approach by showing the high-quality MRI reconstruction of the challenging low-field MR data of the pathology patient, as well as for the simulation study. In future works, we will perform image quality evaluations on large-scale real non-uniform MRI with reader studies.Acknowledgements
No acknowledgement found.References
1. Wang, S., Su, Z., Ying, L., Peng, X., Zhu, S., Liang, F., Feng, D., Liang, D., 2016. Accelerating magnetic resonance imaging via deep learning, in: 2016 IEEE 13th international symposium on biomedical imaging (ISBI), IEEE. pp. 514–517.
2. Schlemper, J., Caballero, J., Hajnal, J.V., Price, A.N., Rueckert, D., 2017. A deep cascade of convolutional neural networks for dynamic mr image reconstruction. IEEE transactions on Medical Imaging 37, 491–503
3. Qin, C., Schlemper, J., Caballero, J., Price, A.N., Hajnal, J.V., Rueckert, D., 2018. Convolutional recurrent neural networks for dynamic mr image reconstruction. IEEE transactions on medical imaging 38, 280–290.
4. Zhou, B., Zhou, S.K., 2020. Dudornet: Learning a dual-domain recurrent network for fast mri reconstruction with deep t1 prior, in: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, pp. 4273–4282.
5. Van Essen, D.C., Smith, S.M., Barch, D.M., Behrens, T.E., Yacoub, E., Ugurbil, K., Consortium, W.M.H., et al., 2013. The wu-minn human connectome project: an overview. Neuroimage 80, 62–79.
6. Lustig, M., Donoho, D.L., Santos, J.M., Pauly, J.M., 2008. Compressed sensing mri. IEEE signal processing magazine 25, 72–82.
7. Schlemper, J., Salehi, S.S.M., Kundu, P., Lazarus, C., Dyvorne, H., Rueckert, D., Sofka, M., 2019. Nonuniform variational network: deep learning for accelerated nonuniform mr image reconstruction, in: International Conference on Medical Image Computing and Computer-Assisted Intervention, Springer. pp. 57–64.
8. Zwart, N.R., Johnson, K.O., Pipe, J.G., 2012. Efficient sample density estimation by combining gridding and an optimized kernel. Magnetic resonance in medicine 67, 701–710.
Figures