3469
DL-MOTIF: Deep Learning Based Motion Transformation Integrated Forward-Fourier Reconstruction for Free-Breathing Liver DCE-MRI1Washington University in St. Louis, Saint Louis, MO, United States
Synopsis
Dynamic contrast-enhanced MRI (DCE-MRI) of the liver offers structural and functional information for assessing the contrast uptake visually. However, respiratory motion and the requirement of high temporal resolution make it difficult to generate high-quality DCE-MRI. In this study, we proposed a novel deep learning based motion transformation integrated forward-Fourier (DL-MOTIF) reconstruction using motion fields derived from a deep learning Phase2Phase (P2P) network and deep learning priors from a residual network on severely undersampled DCE. This approach reconstructs sharp motion-free DCE images with artifacts removal by incorporating deep learning motion fields for motion integration and deep learning priors for regularization.
Introduction
Dynamic contrast-enhanced MRI (DCE-MRI) with high temporal resolution may differentiate between benign and malignant lesions1. Standard-of-care (SOC) DCE is acquired during breath-holding. Free-breathing DCE-MRI techniques have been proposed to minimize respiratory motion artifacts2-5. View-sharing or compressed sensing (CS) with smoothness constrains across adjacent DCE contrasts has been proposed to reduce reconstruction artifacts6,7. However, these techniques may lead to contrast enhancement spill-over. To address this issue, we previously proposed a forward-Fourier motion corrected (FF MoCo) reconstruction incorporating motion derived from a Phase2Phase (P2P) network8 reconstructed 4D MRI9. Recently inspired by the concept in the regularization by artifact removal10, we expanded our FF MoCo reconstruction by including deep learning priors for regularization. The proposed DL-MOTIF method reconstructs sharp and motion-free DCE-MRI with artifact removal on severely undersampled data. 3D motion-corrected DCE images were generated independently for each DCE contrast.Methods
CAPTURE11, a self-navigated respiratory motion detection MR sequence, was utilized to acquire free-breathing DCE images before, during, and after contrast injection (Acq. time: 6min) from four patients using a Siemens 3T MRI scanner. Eovist or Dotarem was injected 30 seconds after the start of the CAPTURE scan. The acquisition parameters were as follows: TE =1.32ms-1.64ms, TR=2.82-3.5ms, FOV=380-450mm2, voxel size=1.25x1.25x3mm3 or 1.4x1.4x3mm3, 64-80 slices, 3200-4000 radial spokes. The continuous free-breathing DCE images were first divided into 34 DCE contrasts with a time interval of 10 seconds. Within each DCE contrast, the k-space data were binned into 5 respiratory phases and reconstructed into 4D respiratory motion-resolved images by P2P8. P2P network was trained using only noisy data. Non-linear registration was performed to obtain 3D deformable MVFs. Next, the DL-MOTIF reconstruction is used to obtain the final 3D dynamic DCE-MRI for each DCE contrast. Motion correction is integrated in the image reconstruction by including a motion transformation. The objective function is shown below:$$I(x,y,z) = \min_{I} \sum_{t} \sum_{i} {\parallel E_{t, i}I - K_{t,i} \parallel}_2^2 + h(I)$$
where the encoding operator Et,i=FtCiMt; Ft is the forward-Fourier (NUFFT) operator for each respiratory phase t; Ci is the coil sensitivity for Coil i; Mt are the MVFs (from P2P 4D images) deforming the image from respiratory Phase 1 to Phase t; I is the 3D motion-corrected image; Kt,i is the acquired k-space data for respiratory Phase t and Coil i. The deep priors were trained based on the RARE framework10. The regularization is:
$$h(I) = \frac{\lambda_{m}}{2} I^{T} (I - N2N(I))$$
where λm is an empirically determined constant. The N2N(I) is a deep learning denoiser on the 3D input image (I) using Noise2Noise (N2N) training strategy10,12. The N2N network was built upon a widely used ResNet architecture13 and it was pre-trained using data without a high-quality ground-truth target from 10 healthy subjects for separating true signal from artifact and noise. Notably, DL-MOTIF is not regularized by smoothness constraints across adjacent DCE contrasts.
The DL-MOTIF reconstruction was repeated for each DCE contrast, resulting in a total of 34 DCE contrasts with a temporal resolution of 10 seconds per DCE contrast. For comparison, two other reconstructions were performed: 1) baseline motion correction method: non-uniform inverse fast Fourier transform (MCNUFFT)14 using a respiratory efficiency window in which 1/3 of the acquisition data close to the end-of-expiration phase were used for the image reconstruction, 2) CS method: CS (using the same 1/3 of the data) with smoothness constraints across adjacent DCE contrasts, similar to commercially available (Siemens) free-breathing DCE reconstructions15. Of note, motion transformation was not performed for either MCNUFFT or CS.
Results
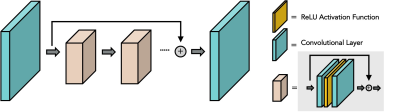
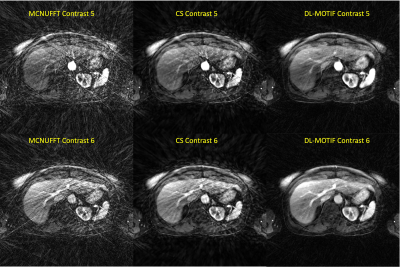
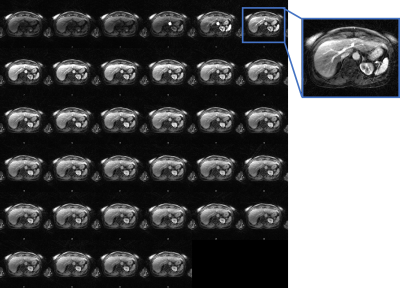
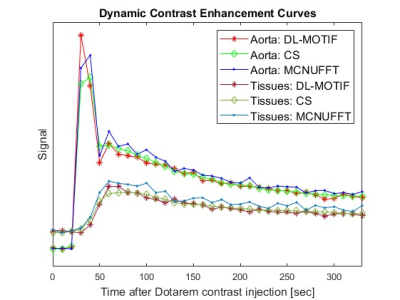
Figure 1 illustrates the network architecture used for training the deep learning priors. Figure 2 shows the reconstructed MCNUFFT, CS and DL-MOTIF images at Contrast 5 and 6 (at arterial phase). MCNUFFT image contains severe streaking artifacts; CS reduced but still suffered from streaking artifacts; DL-MOTIF demonstrated substantial improvement on image quality and artifact removal over both MCNUFFT and CS method. Figure 3 shows the DL-MOTIF images across all 34 DCE contrasts on a patient. These dynamic contrasts allow radiologists to visualized DCE contrasts, including arterial, portal venous, transitional, and 5-min delay phase from a single free-breathing continuous DCE scan. Figure 4 shows the dynamic contrast (Dotarem) enhancement curves on DL-MOTIF, MCNUFFT and CS images at aorta and normal tissues. MCNUFFT showed noisy DCE signal, while CS overly smoothed dynamic signal across adjacent DCE contrasts. DL-MOTIF images effectively removed artifacts and noise, while preserving the DCE contrasts as demonstrated by the sharper arterial signal peak.Discussion and Conclusion
We proposed an DL-MOTIF reconstruction framework that integrates deep learning motion transformation and utilizes a deep learning denoiser as priors. The DL-MOTIF reconstructed sharp and high-quality motion-free images from severely undersampled data –14.9% of the Nyquist sampling rate for a temporal resolution of 10 seconds16. DL-MOTIF allows for free-breathing continuous DCE scan, with no need for breath-hold or a test bolus to determine the exact timing of arterial, portal venous and transitional phases. Furthermore, DL-MOTIF outperforms both the MCNUFFT and CS method (with motion-gating from a respiratory efficiency window) in terms of image-quality and artifact-removal. Unlikely the view-sharing or CS methods, DL-MOTIF method minimizes contrast enhancement spillover from adjacent DCE contrasts observed in (Figure 4). This allows us to derive more accurate dynamic contrast enhancement curves on DL-MOTIF images.Acknowledgements
We acknowledge Siemens Healthineers for providing funding to this study.References
1. Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 2003;17(5):509-520
2. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine 2016;75(4):1484-1498
3. Reiner CS, Neville AM, Nazeer HK, Breault S, Dale BM, Merkle EM, Bashir MR. Contrast-enhanced free-breathing 3D T1-weighted gradient-echo sequence for hepatobiliary MRI in patients with breath-holding difficulties. European radiology 2013;23(11):3087-3093
4. Zhang T, Cheng JY, Potnick AG, Barth RA, Alley MT, Uecker M, Lustig M, Pauly JM, Vasanawala SS. Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. Journal of Magnetic Resonance Imaging 2015;41(2):460-473
5. Ippoliti M, Lukas M, Brenner W, Schaeffter T, Makowski MR, Kolbitsch C. 3D nonrigid motion correction for quantitative assessment of hepatic lesions in DCE-MRI. Magnetic resonance in medicine 2019;82(5):1753-1766
6. Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. Journal of Magnetic Resonance Imaging 2012;36(2):483-491
7. Levine E, Daniel B, Vasanawala S, Hargreaves B, Saranathan M. 3D Cartesian MRI with compressed sensing and variable view sharing using complementary poisson-disc sampling. Magnetic resonance in medicine 2017;77(5):1774-1785
8. Eldeniz C, Gan W, Chen S, Fraum TJ, Ludwig DR, Yan Y, Liu J, Vahle T, Krishnamurthy U, Kamilov US, An H. Phase2Phase: Respiratory Motion-Resolved Reconstruction of Free-Breathing Magnetic Resonance Imaging Using Deep Learning Without a Ground Truth for Improved Liver Imaging. Investigative Radiology 2021. doi:10.1097/rli.0000000000000792
9. Chen S, Eldeniz C, Gan W, Kamilov U, Fraum T, An H. Forward-Fourier Motion-Corrected Reconstruction for Free-Breathing Liver DCE-MRI. Proc Intl Soc Mag Reson Med; 2021. p 0128.
10. Liu J, Sun Y, Eldeniz C, Gan W, An H, Kamilov US. RARE: Image Reconstruction using Deep Priors Learned without Ground Truth. IEEE Journal of Selected Topics in Signal Processing 2020;14(6):1088-1099
11. Eldeniz C, Fraum T, Salter A, Chen Y, Gach HM, Parikh PJ, Fowler KJ, An H. CAPTURE: Consistently Acquired Projections for Tuned and Robust Estimation: A Self-Navigated Respiratory Motion Correction Approach. Investigative radiology 2018;53(5):293-305
12. Lehtinen J, Munkberg J, Hasselgren J, Laine S, Karras T, Aittala M, Aila T. Noise2noise: Learning image restoration without clean data. arXiv preprint arXiv:180304189 2018
13. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition; 2016. p 770-778.
14. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE transactions on signal processing 2003;51(2):560-574
15. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine 2014;72(3):707-717
16. Liang Z-P, Lauterbur PC. Principles of magnetic resonance imaging: SPIE Optical Engineering Press Belllingham, WA; 2000.
Figures