3468
Super-resolution MRI using Novel Slice-profile Based Transformation for Multi-slice 2D TSE Imaging
Jiahao Lin1,2, Miao Qi1,3, Chuthaporn Surawech1,4,5, Steven S Raman1, Holden Wu1, and Kyunghyun Sung1
1Department of Radiology, University of California, Los Angeles, Los Angeles, CA, United States, 2Department of Electrical and Computer Engineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Radiology, The First Affiliated Hospital of China Medical University, Shenyang, China, 4Department of Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, 5Division of Diagnostic Radiology, Department of Radiology, King Chulalongkorn Memorial Hospital, Bangkok, Thailand
1Department of Radiology, University of California, Los Angeles, Los Angeles, CA, United States, 2Department of Electrical and Computer Engineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Radiology, The First Affiliated Hospital of China Medical University, Shenyang, China, 4Department of Radiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, 5Division of Diagnostic Radiology, Department of Radiology, King Chulalongkorn Memorial Hospital, Bangkok, Thailand
Synopsis
Multi-slice two-dimensional turbo spin-echo (2D TSE) imaging is commonly used for its excellent in-plane resolution. However, multiple 2D TSE scans are acquired due to their poor through-plane resolution. In this study, we propose a novel slice-profile-based transformation super-resolution (SPTSR) framework for through-plane super-resolution (SR) of multi-slice 2D TSE imaging. We utilized a large clinical MRI dataset in this study. We demonstrate the effectiveness of our proposed SPTSR framework in 5.5x through-plane SR by both the visual comparison results and reader study results.
Introduction
Multi-slice two-dimensional (2D) turbo spin-echo (TSE) imaging is commonly used because of its high in-plane resolution and relatively short scan time, but its utility is sometimes limited due to poor through-plane resolution 1,2. Thus, multiple 2D TSE scans are acquired in multiple orthogonal imaging planes for comprehensive image interpretations, increasing the overall scan time 2–4. In this study, we propose a novel slice-profile-based transformation super-resolution (SPTSR) framework with deep generative networks for super-resolution (SR) of multi-slice 2D TSE imaging. The networks were trained by synthesized low- resolution (LR) training input of coronal scans via slice-profile-based downsampling (SP-downsampling), and the trained networks inferred on the slice-profile-convolved (SP-conv) testing axial scans input for 5.5x through-plane SR.Methods
Slice-profile Transformations:Previous studies attempted to synthesize LR reformatted training input via the k-space zero-filling (KS-ZF) downsampling. However, the KS-ZF downsampled LR input from the coronal scan is inherently different from its reformatted version of the axial scan. This is because the coronal scan and the axial scan are convolved with their corresponding slice profiles in their through-plane directions. In this study, we propose to apply slice-profile downsampling (SP-downsampling) to the coronal scans for synthesizing LR training input (Figure 1a), and apply slice-profile convolution (SP-conv) to pre-process axial scans for the networks inference input (Figure 1b).
Deep Generative Networks:
The networks architecture was inspired by SRGAN5, with the following three key differences. First, we used three consecutive LR images as input, with the middle slice being the targeted input, to learn the inter-slice spatial relationship. Second, WGAN-GP6 training scheme was used, and thus batch normalizations and the last sigmoid activation function were removed from the discriminator network. Third, the upsampling blocks in the generator model were modified to 1D anisotropic upsampling.
MRI Dataset:
We utilized a total of 3,895 clinical subjects with 4,878 pairs of axial and coronal scans using the multi-slice 2D T2-weighted TSE sequence. Both axial and coronal scans were based on the same imaging sequence except for the imaging-plane orientations. Each scan included 20 slices, with an in-plane resolution of 0.625 × 0.625 mm2. The slice thickness was 3 mm and the slice spacing was 3.6 mm. The training/validation/testing splits were 3,453/392/50 from 3,895 clinical subjects.
Visual Comparisons:
The proposed SPTSR results from T2w-TSE axial scans in the testing dataset were visually compared with the bilinear interpolation, the baseline KS-ZF trained networks, and SMORE7, which is one of the leading self-supervised algorithms for deep learning super-resolution. The corresponding in-plane T2w-TSE coronal scans were served as visual references only, because they were different scans.
Reader Study:
To assess the through-plane SR results, two genitourinary radiologists independently assessed three methods: bilinear interpolation, KS-ZF trained networks (baseline), SPTSR (proposed). In total, 30 test subjects, each with one axial scan, were examined. Three methods were anonymized and randomly shuffled. Along with the visual references (T2w-TSE coronal scans), all four image sets were loaded into OsiriX (Pixmeo SARL, Bernex, Switzerland), and the readers scrolled through the coronal slices. Diagnostic quality metrics of sharpness, artifacts, noise level and overall image quality of each method were scored on a 4-point Likert scale (1 is the worst and 4 is the best). The readers also blindly ranked the overall quality of the three methods. Averaged ratings and rankings from two readers were compared between three methods. Mann-Whitney U tests were used to assess the significant differences (p<0.01) between the three methods. Cohen’s Kappa was calculated for inter-reader variability8.
Results
Visual Comparisons:The examples of the testing results are shown in Figure 2 for visual comparison. Compared to the other methods, the proposed SPTSR output was much less noisy, much sharper, and showed finer detail structure, similar to the visual reference images (T2w-TSE coronal scan).
Reader Study:
The reader study results are as demonstrated in Figure 3. The proposed SPTSR method reaches an overall image quality score of (3.72 ± 0.44), and 20 cases out of 30 received an excellent score (4.0) from both readers. The overall image quality scores from two readers show the decisive advantage of the proposed SPTSR method compared to the other two methods (the proposed method: 29 cases have the best quality; the baseline method: 1 case has the best quality; the BI method: all 30 cases have the worst quality). The ranking results from the two readers are perfectly matched. The proposed SPTSR method is significantly better in all categories compared to the baseline KS-ZF trained network in terms of sharpness, artifact, noise level, and overall image quality (p<0.01). The score results from two readers show substantial agreement with Cohen’s Kappa of 0.68 (95% confidence interval: 0.62-0.74).
Discussions
We proposed a novel slice-profile transformation-based super-resolution (SPTSR) framework for multi-slice 2D TSE MRI. We utilized a large dataset of clinical subjects and scans and demonstrated the visual improvements for 5.5x through-plane SR compared to the k-space zero-filling-based method and the super-resolution algorithm SMORE7. Our reader study results confirm the superiority of SPTSR compared to the KS-ZF trained networks.Conclusions
We developed a novel SPTSR framework for super-resolution of multi-slice 2D TSE MRI. The visual results and reader study results demonstrated the effectiveness of our proposed framework in 5.5x through-plane SR.Acknowledgements
This work was supported by the National Institutes of Health (NIH) R01-CA248506 and funds from the Integrated Diagnostics Program, Department of Radiological Sciences & Pathology, David Geffen School of Medicine at UCLA.References
- Greenspan H. Super-resolution in medical imaging. Comput J. 2009;52(1):43-63. doi:10.1093/COMJNL/BXM0752.
- Sood R, Rusu M. Anisotropic super resolution in prostate mri using super resolution generative adversarial networks. Proc - Int Symp Biomed Imaging. 2019;2019-April(Isbi):1688-1691. doi:10.1109/ISBI.2019.87592373.
- Kijowski R, Davis KW, Woods MA, et al. Knee joint: Comprehensive assessment with 3D isotropic resolution fast spin-echo MR imaging - Diagnostic performance compared with that of conventional MR imaging at 3.0 T. Radiology. 2009;252(2):486-495. doi:10.1148/radiol.25230900284.
- Torrents-Barrena J, Piella G, Masoller N, et al. Fully automatic 3D reconstruction of the placenta and its peripheral vasculature in intrauterine fetal MRI. Med Image Anal. 2019;54:263-279. doi:10.1016/J.MEDIA.2019.03.0085.
- Ledig C, Theis L, Huszár F, et al. Photo-Realistic Single Image Super-Resolution Using a Generative Adversarial Network. Cvpr. 2017;2(3):4.6.
- Gulrajani I, Ahmed F, Arjovsky M, Dumoulin V, Courville A. Improved training of wasserstein GANs. Adv Neural Inf Process Syst. 2017;2017-Decem:5768-5778.7.
- Zhao C, Dewey BE, Pham DL, Calabresi PA, Reich DS, Prince JL. SMORE: A Self-Supervised Anti-Aliasing and Super-Resolution Algorithm for MRI Using Deep Learning. IEEE Trans Med Imaging. 2021;40(3):805-817. doi:10.1109/TMI.2020.3037187
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. 2012;22(3):276.
Figures
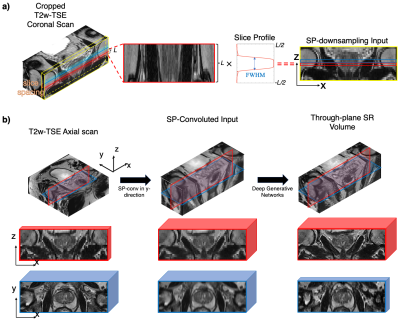
Figure 1. a) The proposed SP-downsampling method. Each line of pixels is acquired by multiplying the slice profile of length L, to the same physical location on the cropped coronal scan; b) The proposed SR inference flow. T2-TSE axial scan is cropped and SP-convoluted in y-direction to prepare the convoluted input, which is then fed through the deep generative networks to generate through-plane SR volume. Red patches represent coronal views and blue patches represent axial views. Patch thickness represents the voxel thickness in the through-plane of the patch.
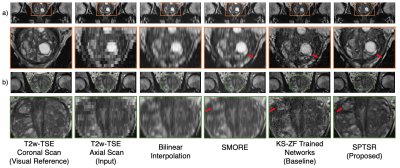
Figure 2. The through-plane SR testing results with reformatted T2w axial scan input. a) and b) represents two images slices from two different testing subjects. From left to right: The T2w coronal scan of the subject, as a visual reference; the testing reformatted T2w axial scan as the inference input; the bilinear interpolation of the input; the inference output of SMORE; the baseline inference output with KS-ZF trained networks; the proposed SPTSR inference output with SP-downsampling trained network, and SP-convoluted inference input.
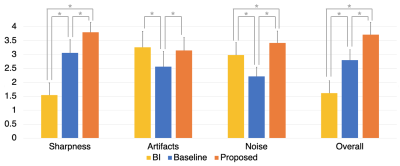
Figure 3. Two radiologists qualitatively assessed the diagnostic quality of Bilinear Interpolation (BI), KS-ZF trained network (baseline), SPTSR (proposed), for sharpness, artifacts, noise and overall image quality on a 1 to 4 scale (higher the better). The ratings were averaged from two readers. Error bar represents the standard deviations. Mann-Whitney U tests assessed whether the average scores were significantly different among three groups. *p<0.01
DOI: https://doi.org/10.58530/2022/3468