3455
Using Low Rank Plus Sparse (L+S) Reconstruction to Accelerate Dynamic Hyperpolarized 13C Spiral Chemical Shift Imaging In Vivo1University of Maryland, Baltimore, Baltimore, MD, United States
Synopsis
The goal of this study is to validate the Low Rank Plus Sparse Reconstruction algorithm in in-vivo spectroscopic imaging applications. The proposed method can be used to increase temporal and/or spatial resolution of dynamic hyperpolarized 13C imaging without compromising image quality.
Introduction
Under-sampled dynamic MRI data can be successfully reconstructed via the recently developed low rank (L) plus sparse (S) matrix decomposition model (L+S) without significant loss of information [1,2], thus providing the possibility to increase temporal resolution of dynamic imaging. To use the method for spectroscopic imaging, we can either iterate the L+S algorithm for the spatiotemporal matrix at each frequency point independently [3] or directly iterate on the spectro-spatio-temporal matrix via the 3D NuFFT operator [4]. For the later method, previous work has shown its successful application with retrospectively undersampled data. In this work, we present its application in truly accelerated hyperpolarized C13 imaging with a pseudo-randomly undersampled spiral chemical shift imaging data acquisition scheme.Methods
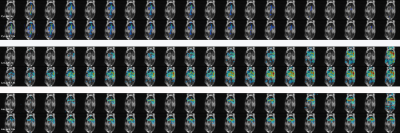
Hyperpolarized C13 spiral chemical shift imaging (spCSI) was performed on a healthy mouse using clinical 3T GE MR scanner (GE Healthcare, Waukesha, WI, USA) with a 1H-13C dual-tuned RF coil. The animal received a 1µmol/g dose of 125mM hyperpolarized pyruvate (approximately 0.23 mL) injected over 6 seconds through a tail vein catheter. Scan parameters for the 2D dynamic spCSI sequence was: 60 × 60 mm FOV, 2 × 2 mm2 nominal in-plane resolution, 10mm slice thickness in coronal orientation centered at the two kidneys. The fully sampled spiral k-space trajectory was comprised of 24 interleaves, with 24 echoes and a spectral width of 280Hz. The spCSI sequence was carried out in such a way: one fully sampled block of 24 interleaves were carried out for every 3 second interval. The entire scan has 10 fully sampled blocks, or equivalently 24x10=240 excitations for a total scan time of 30 seconds. The order of the interleaves was randomly permuted for each block. Thus, dynamic images with 10 time points, 3 second temporal resolution can be reconstructed using the fully sampled blocks, whereas dynamic images with 20 time points, 1.5 second temporal resolution can be reconstructed using every 12 interleaves (50% under-sampled). Fig.1 illustrates the generation of dynamic images at two temporal resolutions using the same data acquired. A variable flip angle scheme was used for the entire scan. The scan started 3 seconds after the beginning of injection. The prospectively under-sampled dataset was reconstructed via the L+S algorithm similar to previous retrospective study [4]. Metabolic images of pyruvate, lactate, and alanine were calculated by phasing the spectrum in each voxel and integrating the resulting peak from the spectro-spatial-temporal matrix after the L+S iterations. λL , λS and the tolerance limit for the L+S reconstruction were 0.01, 0.001 and 0.02 respectively, selected to optimize the image quality with least distortion to dynamic evolvements of each metabolite.Results and Discussion
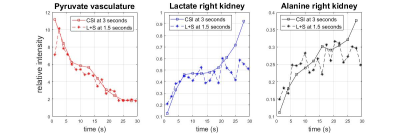
Reconstruction results at two temporal resolutions are shown in Fig.2. Mean intensity in the vasculature and right kidney region are calculated for pyruvate, lactate and alanine respectively, plotted in Fig.3. The L+S algorithm successfully reconstructed the 50% under-sampled dataset, with no significant artifacts seen for all three metabolites. Comparing the dynamic curves for the respective regions proves that the L+S reconstruction can recover the dynamics at a higher temporal resolution (acceleration factor of 2 in this experiment). The rise of lactate and alanine intensity towards the end of the scan can be contributed to the utilization of variable flip angle with accumulation of product metabolites along the time course. Slight deviation in the dynamic curve can be observed for lactate and alanine, since the pyruvate is the dominant metabolite in terms of signal intensity, and the L+S algorithm is enforcing low rank property in between the time points when operating on the entire spectro-spatio-temporal matrix. Further finetuning of the iteration parameters can possibly get a better estimation of the true dynamics.Conclusion
An acceleration factor of 2 in temporal resolution is achieved for in vivo Hyperpolarized 13C Spiral Chemical Shift Imaging using Low Rank Plus Sparse Reconstruction algorithm.Acknowledgements
This work was supported by NIH grants R21 NS096575, R01 DK106395, R21 CA213020, and R21 CA202694.References
[1] Otazo, et al. "Low‐rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components." Magnetic resonance in medicine 73.3 (2015): 1125-1136.
[2] Milshteyn, et al. "Using a local low rank plus sparse reconstruction to accelerate dynamic hyperpolarized 13C imaging using the bSSFP sequence." Journal of Magnetic Resonance 290 (2018): 46-59.
[3] DeVience, et al. "Speeding up dynamic spiral chemical shift imaging with incoherent sampling and low‐rank matrix completion." Magnetic resonance in medicine 77.3 (2017): 951-960.
[4] Zhu, et al. "Accelerating Hyperpolarized 13C Spiral Chemical Shift Imaging with Joint Spectral-Spatial Low Rank Plus Sparse Reconstruction." Proc. Intl. Soc. Mag. Reson. Med. 29 (2021).
Figures