3435
Multimodal MRI of cervical spinal cord injury before and after surgical myelotomy1Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Surgical myelotomy, a procedure intended to relieve pressure on an acutely-injured spinal cord, improves long-term functional and pathologic outcomes in animal models. However, the direct consequences of myelotomy on the cord pathophysiology have not been evaluated in the acute setting. In this work, we used multimodal MRI tailored for the spinal cord to monitor changes in edema, hemorrhage, axonal injury, and perfusion immediately before and 24 hours after surgical myelotomy. The results demonstrate spatial and temporal changes and provide unique insight into the pathophysiology of acute injury and its intervention.
Introduction
Myelotomy is a surgical incision into the spinal cord along its longitudinal dorsal length that aims to relieve intramedullary pressure and reduce hemorrhage. In prior animal studies of thoracic spinal cord injury (SCI), myelotomy improves long-term functional and pathologic outcomes. However, it has not been evaluated in cervical SCI which is the most common level of injury in SCI patients, nor has it been coupled with noninvasive imaging to reveal early indications of success in limiting damage. In this study, we evaluated the consequences of myelotomy using pre- and post-operative multimodal MRI in a rat model of cervical SCI.Methods
Experimental Design: Twenty-seven Sprague-Dawley rats (8- to 12-week-old, 275-300g) were used with equal male/female ratios. A range of moderate to severe contusion injury was induced in the cervical cord at C5. Rats underwent a second survival surgery with myelotomy or sham-myelotomy at 4 hours post injury. Myelotomy was a 3 mm longitudinal midline incision at a depth of 1 mm into the cord. A total of three groups were compared: SCI+sham (n=14), SCI+myelotomy (n=9), and Sham+myelotomy (n=4). The Sham+myelotomy group served as a control examine the effects of myelotomy surgery on its own. Pre-operative MRI was performed 2 hours post-injury SCI/sham followed immediately by myelotomy/sham at 4 hours post injury. Post-operative MRI was performed at 24 hours post injury.Magnetic Resonance Imaging: A Bruker BioSpec 9.4T animal system was used with a 38mm diameter Litz volume coil. All imaging was acquired on a sagittal midline slice. T2w imaging used a rapid acquisition with relaxation (RARE) with TR/TE=2000/20, 59, 99ms, resolution=156x156mm2, slice thickness=1mm. Steady-state 3D T2*w imaging used a multi-gradient echo sequence with TR/TE=65/2.5ms, FA=14, resolution=224mm3, echo spacing=2.9ms, and 6 echoes. A diffusion-prepared RARE was used with TR/TE=1400/4.29ms, resolution=200mm2, b┴=2000s/mm2, and b||=0 or 800s/mm2 with 24 directions. Perfusion MRI used a pseudocontinuous arterial spin labelling (pCASL) slice-matched to DWI with TR/TE=4026/4.95ms, labeling duration=1100ms, and post label delay=100-400ms.
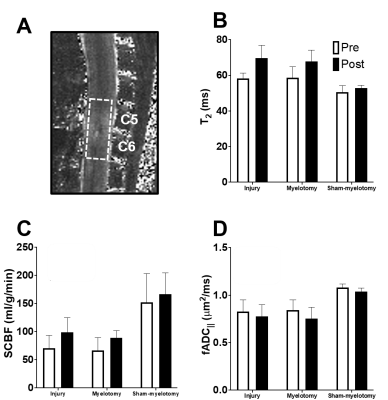
Data Analysis: Maps of T2, parallel ADC (fADC||), and spinal cord blood flow (SCBF) were calculated as described previously1–3. For quantification, a single region of interest (ROI) encompassing C5 and C6 was drawn manually for each dataset to obtain mean values.
Results
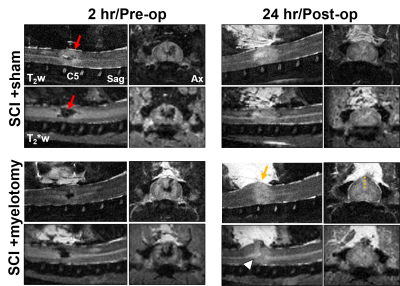
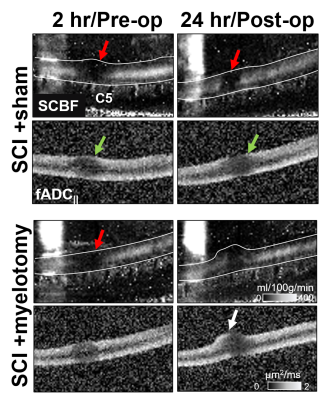
Representative images reveal the effects of contusion injury and its evolution over 2 to 24 hours along with the effects of myelotomy. Edema (T2w) and hemorrhage (T2*w) were prominent acutely, with hemorrhage seemingly decreasing by the 24 hour timepoint. The effect of myelotomy on the cord morphology revealed its expansion out of the spinal canal at the incision site (Fig.1B).A prominent deficit in SCBF was also observed after injury that diminished in size without intervention (Fig.2). No clear changes were evident due to the effect of myelotomy in the SCI+myelotomy animal compared to the SCI+sham animal. Comparatively, the DWI-lesion appeared to increase in size between the 2 and 24 hour timepoints without intervention. Interestingly, in the SCI+myelotomy animal, a portion of the cord protruding from the myelotomoy incision appeared to have normal diffusion characteristics (Fig.2 white arrow). Qualitatively, it was unclear whether myelotomy altered the degree or extent of diffusion changes.
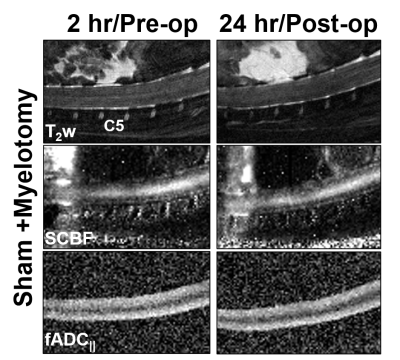
To ensure the myelotomy procedure did not cause damage on its own, myelotomy was performed on sham-injured animals (Fig.3), all indicating there was no abnormalities detected on any of the contrasts. To quantify the changes across all animals, a region of interest over C5 and C6 was used for mean cord values (Fig.4). Although the effects of SCI were evident and characteristic of prior studies, no significant differences were found between the SCI+sham and SCI+myelotomy groups.
Discussion
The subacute evolution of cervical contusion SCI and the effects of myelotomy were monitored by multimodal MRI tailored for the spinal cord. Notably, a spatial and temporal evolution of diffusion and perfusion contrasts was observed in SCI. A considerable perfusion deficit was evident at 2 hours and diminished by 24 hours. Myelotomy was expected to relieve intramedullary pressure4,5, and it caused the spinal cord to swell out through the incised dura. No further edema or hemorrhage was caused by myelotomy, but it was uncertain whether myelotomy resulted in more subtle changes that were undetectable by using a large whole-cord region of interest. A more comprehensive analysis is needed to account for the spatiotemporal evolution of the spinal cord.Conclusion
This study described the progression of cervical contusion SCI within 24 hours of injury and the effects of surgical myelotomy. The effect of myelotomy on cervical spinal cord injury was visualized with multimodal MRI, including T2w, T2*w, DWI and pCASL. A spatial mismatch and temporal differences in anatomical, microstructural, and vascular injury were observed.Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Spinal Cord Injury Research Program (W81XWH-19-SCIRP-IIRA) and by the National Institutes of Neurological Disorders and Stroke (NS109090). We thank Matthew Runquist and Qian (Kathleen) Yin for experimental assistance.References
1. Lee, S.-Y., Meyer, B. P., Kurpad, S. N. & Budde, M. D. Diffusion-prepared fast spin echo for artifact-free spinal cord imaging. Magn. Reson. Med. 86, 984–994 (2021).
2. Briana Meyer; Lydiane Hirschler; Jan Warnking; Emmanuel Barbier; Matthew Budde. Preclinical Spinal Cord Perfusion Imaging with Pseudo-Continuous Arterial Spin Labeling. ISRMR SMRT Virtual Conf. (2020).
3. Buxton, R. B. et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 40, 383–396 (1998).
4. Koenig, M. A. Cerebral Edema and Elevated Intracranial Pressure. Contin. Minneap. Minn 24, 1588–1602 (2018).
5. Zoerle, T. et al. Intracranial Pressure After Subarachnoid Hemorrhage*. Crit. Care Med. 43, 168–176 (2015).
6. Meng, X., Fisher, M., Shen, Q., Sotak, C. H. & Duong, T. Q. Characterizing the Diffusion/Perfusion Mismatch in Experimental Focal Cerebral Ischemia. Ann. Neurol. 55, 207–212 (2004).
Figures