3434
Fluorine-19 MRI of Stem Cell-Derived Alveolar-Like Macrophages Tagged with Perfluoropolyether
Janny Yeyoung Kim1,2, Michael Litvack1, Daniel Li1, Brandon Zanette1, Martin Post1, and Giles Santyr1,2
1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada
1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
When stem cell-derived alveolar-like macrophages (ALMs) are labeled with perfluoropolyether (PFPE), they can potentially be monitored and tracked longitudinally in vivo using 19F MRI. In this work, different concentrations of PFPE and 5 million PFPE-labeled ALMs were imaged in vitro using 19F MRI at 3T. The results demonstrated the feasibility of detecting a small number of PFPE-labeled ALMs. This work will provide guidance for the upcoming in vivo instillation experiments in rat lungs. Longitudinal in vivo studies will further aid ALMs to be clinically translated as a stem cell therapy to treat chronic lung diseases.
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease diagnosed in ventilated preterm infants. In BPD patients, altered innate immune activation and inflammatory signaling inhibit branching morphogenesis in the lungs1. Stem cell-derived alveolar-like macrophages (ALMs) have been shown to treat similar lung injury in animal models by promoting gas exchange and tissue repair2. For ALMs to be used as therapeutics, a non-invasive imaging modality to guide treatment is essential. Magnetic Resonance Imaging (MRI), such as Fluorine-19 (19F) MRI, is a non-invasive imaging technique, suitable for longitudinal monitoring of cells. Cells can be labeled with fluorinated compounds, such as perfluoropolyether (PFPE) to potentially make them visible in the lung with no background signal3. Furthermore, 19F MRI allows quantitation of the precise number of 19F-labeled cells per voxel4, important for the eventual use of ALMs for clinical therapies. In preparation for longitudinal in vivo studies, in vitro 19F MRI was performed in different dilutions of PFPE, as well as PFPE-labeled ALMs to mimic in vivo instillation experiments in rat lungs. 1H images were also obtained to confirm registration of the 19F images.Methods
A PFPE-based reagent (120mg/mL) was used for 19F labeling and detection (CS-1000, CelSense Inc.). Varying concentrations of PFPE, mixed with phosphate-buffered saline (PBS) while maintaining a consistent 1mL total volume, were prepared in Eppendorf tubes: 100%, 70%, 40%, 20%, 8%, 4%, 2%, 1%. For example, 70% PFPE would correspond to 700μL of PFPE reagent with 300μL of PBS. The aligned tubes were adhered to the side of a 500mL bottle containing mineral oil to mimic the loading conditions of a rat inside the coil. 19F MRI was performed using a 3T clinical scanner (Philips Achieva) with a custom-built transmit-receive animal sized birdcage coil tuned to the 19F frequency. Imaging was done using a gradient echo pulse sequence (see parameters shown in Table 1). A human knee coil was used for proton MRI. Images and signal-to-noise ratio (SNR) of eight different PFPE solutions were calculated using ImageJ and MATLAB. Relative SNR was calculated where each SNR was divided by SNR from the stock solution (100%) and multiplied by 100. ALMs were produced and cultured following the protocols of Litvack et al2 and Riberdy et al5 respectively. The ALMs were co-incubated with 8% PFPE for four hours. 5x106 PFPE-labeled cells were harvested (manually counted). Cells were spun down in a pellet form in 1mL of PBS. The fluorine uptake by ALMs previously measured was used to estimate PFPE uptake by the cell pellet. For 5 million cells, it was determined approximately 18μL of PFPE would be internalized, corresponding to 1.8% concentration.Results
Figure 1 shows the SNRs measured from the eight different concentrations of PFPE solutions. The relationship between the relative SNR and PFPE concentration showed the expected linear trend (slope of 1.002 and r-squared value of 0.992). The value of relative SNR measured from the PFPE-labeled cell pellet is also shown in Figure 1 in blue. When the cell pellet was imaged, the linear relationship still held true (slope of 1.009). Figure 2 shows the overlay image of the cell pellet with three different PFPE concentrations: 100%, 20% and 4%. The absolute SNRs were 1091, 212, 36.6 for 100%, 20% and 4% tubes respectively and 7.37 for the cell pellet.Discussion
The linear relationship between SNR and PFPE concentration (Fig. 1) confirms that visualization of PFPE down to 10μL (1% PFPE) is feasible using 19F MRI at 3T. This work further demonstrates feasibility of detecting a typical number of cells expected to be used for stem cell treatment in rats (ie. 5x106 cells). Previous cell imaging studies have been performed with specialized pulse sequences such as balanced steady-state free precession6 or rapid acquisition relaxation enhancement7, and at much higher static magnetic field strengths8,9. This study is distinctive since the imaging was done at a typical clinical field strength and with a conventional pulse sequence and parameters. Although imaging 5x106 PFPE-labeled ALMs was successful in vitro, signal from PFPE-labeled cells fell below signal from PFPE solutions (Fig. 1) potentially due to difference in relaxation properties in liquid versus pellet. Detection of cells can be improved by using specialized receive coils, further manipulating imaging parameters, such as number of averages or by instilling more cells. The results confirmed the feasibility of imaging fluorine-labeled ALMs with loading condition of 8% PFPE with four-hour co-incubation (88% cell viability). Future work including in vivo instillation experiments with 10 million PFPE-labeled ALMs is underway. On top of the MRI experiments, two-photon tomography (TissueVision, Inc.) will be performed on the excised lung specimens to further validate the in vivo localization of the instilled ALMs.Conclusion
Various concentrations of PFPE solutions and 5 million PFPE-tagged ALMs were imaged using 19F MRI in vitro. This work serves as a preliminary step toward in vivo MRI of ALMs instilled in the rat lung. Following successful detection of PFPE-labeled ALMs in rat lungs, considering the advantages 19F MRI gives, longitudinal monitoring of biodistribution of ALMs may be possible. This will further benefit the clinical translation of ALMs as a stem cell therapy to treat chronic lung diseases, including BPD.Acknowledgements
No acknowledgement found.References
- Blackwell, T. S., Hipps, A. N., Yamamoto, Y., Han, W., Barham, W. J., Ostrowski, M. C., … Prince, L. S. (2011). NF-κB Signaling in Fetal Lung Macrophages Disrupts Airway Morphogenesis. The Journal of Immunology, 187(5), 2740–2747.
- Litvack, M. L., Wigle, T. J., Lee, J., Wang, J., Ackerley, C., Grunebaum, E., & Post, M. (2016). Alveolar-like Stem Cell–derived Myb−Macrophages Promote Recovery and Survival in Airway Disease. American Journal of Respiratory and Critical Care Medicine, 193(11), 1219–1229.
- Fox, M. S., Gaudet, J. M., & Foster, P. J. (2015). Fluorine-19 Mri Contrast Agents for Cell Tracking and Lung Imaging. Magnetic Resonance Insights, 8s1, MRI.S23559.
- Makela, A. V., Gaudet, J. M., & Foster, P. J. (2017). Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Scientific Reports, 7(1).
- Riberdy, V., Litvack, M., Stirrat, E., Couch, M., Post, M., & Santyr, G. E. (2019). Hyperpolarized 129 Xe imaging of embryonic stem cell‐derived alveolar‐like macrophages in rat lungs: proof‐of‐concept study using superparamagnetic iron oxide nanoparticles. Magnetic Resonance in Medicine, 83(4), 1356–1367.
- Ribot, E. J., Gaudet, J. M., Chen, Y., Gilbert, K. M., & Foster, P. J. (2014). In vivo MR detection of fluorine-labeled human MSC using the bSSFP sequence. International Journal of Nanomedicine, 9(1), 1731–1739.
- Rizzo, S., Padelli, F., Rinaldi, E., Gioeni, D., Aquino, D., Brizzola, S., Acocella, F., Spaggiari, L., Baggi, F., Bellomi, M., Bruzzone, M. G., & Petrella, F. (2020). 7-T MRI tracking of mesenchymal stromal cells after lung injection in a rat model. European Radiology Experimental, 4(1).
- Constantinides, C., Maguire, M., McNeill, E., Carnicer, R., Swider, E., Srinivas, M., Carr, C. A., & Schneider, J. E. (2018). Fast, quantitative, murine cardiac19F MRI/ MRS of PFCE-labeled progenitor stem cells and macrophages at 9.4T. PLoS ONE, 13(1), 1–21.
- Makela, A. V., & Foster, P. J. (2018). Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magnetic Resonance in Medicine, 80(3), 1138–1147.
Figures
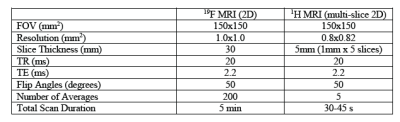
Table 1. Scan parameters used for in vitro 19F MRI and 1H MRI. Proton MRI was obtained for structural
information of the subjects in 19F images.
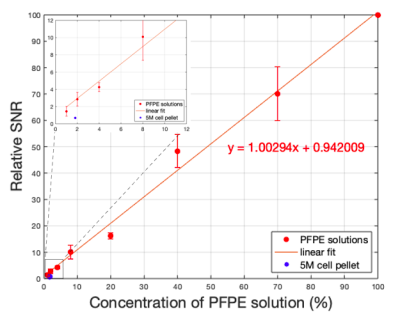
Figure 1. Average ± standard deviation of relative SNR plotted as a function of PFPE solution concentration (N=3). The equation of the linear fit is also shown. The relative SNR of the 5 million PFPE-labeled cell pellet is also shown in blue. Inset: The plot was zoomed-in on lower concentrations (1%, 2%, 4% and 8%), showing the closer look at where the cell pellet datapoint is located.
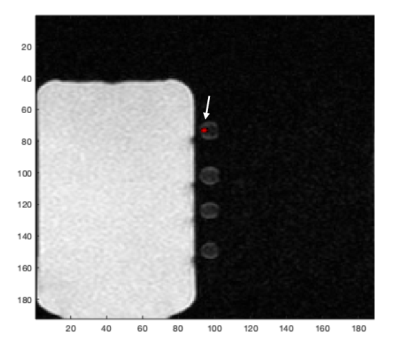
Figure 2. An overlay of 19F image (red) and the 1H image (grey-scale) of three PFPE solutions (bottom)
and a PFPE-labeled cell pellet (top). Due to differences in window/levelling, only the fluorine signal
coming from the cell pellet is shown, as indicated by the arrow.
DOI: https://doi.org/10.58530/2022/3434