3425
Dynamic Spiral Imaging of Rodent Pulmonary Ventilation using Natural-Abundance Hyperpolarized 129Xe Imaging1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
While a hyperpolarized 129Xe MRI-derived fractional ventilation is a valuable metric for assessing lung function, currently implemented schemes use enriched xenon and rely on static images acquired over breath holds to derive FV. In order to probe pulmonary ventilation under more physiologically natural conditions while keeping costs low, we acquired images during continuous controlled breathing in a mechanically ventilated rat using natural-abundance xenon. In addition to FV, we were able to derive the additional physiologically relevant metrics of tidal volume, functional residual capacity, and phase.
Introduction
Hyperpolarized 129Xe (HXe) MRI-derived measurements of pulmonary ventilation are incredibly useful in both pre-clinical and clinical studies for probing the underlying mechanisms driving disease onset and progression. The commonly used fractional ventilation (FV) is an especially useful quantitative metric for assessing the severity of diseases such as chronic obstructive pulmonary disease [1], cystic fibrosis [2], and lung cancer [3]. However, FV maps are typically derived from static HXe images acquired at end-inspiration (EI) breath holds and, depending on the number of HXe wash-in breaths used, can obscure the heterogeneous, non-static physiological behavior that occurs during normal breathing (air trapping, etc.) [4]. Moreover, such FV measurements are typically acquired using enriched HXe, which makes frequent application in animal studies infeasible due to its high cost. In this work, we demonstrate the feasibility of measuring FV, along with other physiologically relevant parameters, via continuous image acquisition during breathing using natural-abundance HXe.Methods
All animal studies and protocols were approved by the University’s Institutional Animal Care and Use Committee. One healthy male Sprague-Dawley rat (330g) was anesthetized, intubated, mechanically ventilated using a home-built ventilator with either air or a HXe gas mixture (30% O2, 70% N2/HXe) at tidal volume of 10 ml/kg, and imaged in a horizontal-bore 3T animal research magnet (Bruker BioSpec 3T). 1.5L of natural-abundance xenon was polarized using a prototype commercial optical pumping system (XeBox-E10, Xemed LLC, NH). 1H T2-weighted fast spin-echo images were acquired for localization, and a 2D spiral sequence was used to acquire images during continuous breathing at a rate of approximately ~30 BPM until the source HXe was emptied (matrix size = 64´64, slices = 10, slice thickness = 2.7mm, no slice gap, FOV = 50mm´50mm, interleaves = 10, receiver bandwidth = 75 kHz, TE/TR = 1.391ms/14.03ms, flip-angle = 5°, points = 390, pulse length = 0.84ms, pulse shape = Calculated, ~15.2 minutes imaging time). Images were reconstructed offline using custom software developed in MATLAB (MathWorks, Inc., Natick, MA). Briefly, the signal intensity of the first five points of every interleave FID was plotted as function of time and a breath-to-breath time was determined; this time was then subdivided by the number of desired bins (12), and interleaves were placed in their respective bins and averaged over the number of ~650 averages acquired. Images were then registered both in-plane and across slices to account for intra- and inter-slice movement using the ANTs registration toolbox, and were subsequently fit to a model of signal decay and replenishment to derive FV:$$$ M_{j+1} = [M_{j}+M_{inf}(v_{j+1}-v_{j})]e^{-Γ(t_{j+1}-t_{j})} $$$
$$$ M_{j+1} = M_{j}(\frac{v_{j+1}}{v_{j}})e^{-Γ(t_{j+1}-t_{j})} $$$
where the first equation represents source magnetization entering a voxel during inhalation at breath j+1 with residual magnetization from breath j, and the second equation represents signal decay from both exhalation and O2 and RF-induced magnetization loss. Variables v and t represent entering/leaving gas volumes and times at the jth or (j+1)th breath, respectively.
Results
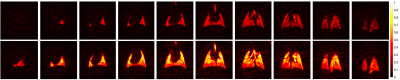
Figure 1 shows spin-density images at two phases, end-expiration (EE) and EI. The largest differences between EE and EI images are a considerable reduction in lung size and signal intensity, which is to be expected. Moreover, a considerable degree of inter-slice motion is seen in the lung parenchyma and is particularly accentuated in the first four (anterior) slices. Figure 2 shows fit result maps: average signal intensity (top row), TV (second row), FRC (third row), FV (fourth row), and phase (fifth row).Discussion
The move to spiral imaging during continuous breathing offers two primary advantages over traditional wash-in/wash-out breath-hold imaging for measuring FV. First, we can acquire images using natural-abundance HXe and achieve comparable signal-to-noise to wash-in/wash-out images by averaging over the course of 15 – 20 minutes; wash-in/wash-out images generally utilize enriched HXe, so the use of natural-abundance HXe greatly reduces the cost of measuring FV, making it more practical for animal studies. Second, imaging during continuous breathing allows us to capture the full extent of ventilation heterogeneity that occurs during normal breathing as well as in the case of disease. While the animal imaged in this study was healthy, it nevertheless served as a demonstration of the feasibility of acquiring images with adequate signal-to-noise that yield plausible maps of FV, TV, FRC, and phase.Conclusion
Imaging during continuous breathing with natural-abundance HXe can be used derive a variety of physiologically relevant parameters of pulmonary ventilation .Acknowledgements
No acknowledgement found.References
[1] Hamedani, Hooman, et al. "A hybrid multibreath wash‐in wash‐out lung function quantification scheme in human subjects using hyperpolarized 3He MRI for simultaneous assessment of specific ventilation, alveolar oxygen tension, oxygen uptake, and air trapping." Magnetic resonance in medicine 78.2 (2017): 611-624.
[2] Couch, Marcus J., et al. "Assessing the feasibility of hyperpolarized 129Xe multiple‐breath washout MRI in pediatric cystic fibrosis." Magnetic resonance in medicine 84.1 (2020): 304-311.
[3] Loza, Luis A., et al. "Quantification of Ventilation and Gas Uptake in Free-Breathing Mice With Hyperpolarized 129 Xe MRI." IEEE transactions on medical imaging 38.9 (2019): 2081-2091.
[4] Hamedani, H., et al. "A Comparison of Hyperpolarized Gas Imaging of Aeration and Fractional Ventilation." C108. COPD: PHENOTYPE, MECHANISM, AND TREATMENT. American Thoracic Society, 2019. A5772-A5772
Figures