3423
In Vivo Oxygen Imaging of Implanted Islet Encapsulation Devices1O2M Technologies, LLC, Chicago, IL, United States, 2Radiation and Cellular Oncology, The University of Chicago, Chicago, IL, United States
Synopsis
The lack of oxygen supply to the highly metabolic pancreatic islet cells is one of the major factors contributing to the failure of islet transplantation devices designed to cure type I diabetes (T1D). Several approaches to improve oxygenation in these devices have been developed. However, the lack of available technologies to provide reliable pO2 assessment in and around devices hinders the progress severely. We performed in vivo oxygen imaging of cell loaded encapsulation devices using pulse electron paramagnetic resonance oxygen imaging (EPROI) technique. For the first time, whole-body EPROI is used to monitor pO2 of implanted devices in this work.
Target Audience
Members of the MR community and physicians involved in type I diabetes (T1D) therapy development, cancer research, targeted drug delivery, EPR instrumentation, pulse sequence and technology development.Purpose
The purpose of this work is to develop methods and protocols to image partial oxygen pressure (pO2) of implanted islet encapsulation devices in mouse model. The lack of oxygen supply to the highly metabolic pancreatic islet cells is one of the major factors contributing to the failure of islet transplantation devices targeting the cure of type I diabetes (T1D). The loss of islets due to hypoxia is common in almost all modes of islet transplantation – from micro-encapsulation to macro-encapsulation devices and tissue-grafts. Several approaches to improve oxygenation in these transplantation devices have been tested (1-6). However, the lack of available technologies to provide reliable oxygen partial pressure (pO2) assessment in and around devices hinders the progress severely. With the support of JDRF, an “Oxygen Measurement Core” facility was established at O2MTM Technologies in 2019. We performed in vivo oxygen imaging of cell loaded encapsulation devices in the core using pulse electron paramagnetic resonance oxygen imaging (EPROI) technique (7,8). In the current work, we present a four-week pO2 imaging study of cell loaded TheraCyte devices.Materials and Methods
Devices: Commercially available 20 mL TheraCyte cell encapsulation devices (n=3) were used for the experiments (4).Animals and cells: Al experiments were performed under the approved protocol from UIC’s IACUC committee. Islets were isolated from 250 g male Sprague Dawley rat using Clzyme RI (VitaCyte, 005-1030) and the Histopaque (SigmaAldrich, 10771 and 11191) gradient method. Five hundred IEQ rat islets were seeded in each TheraCyte device. The devices were implanted in 8 weeks old male C57BL6 mice (one per animal, n=3) subcutaneously on dorsal side. Weekly MRI (for anatomical mapping of the implanted devices) and EPROI (for oxygen mapping) were performed for four weeks.
MRI: Anatomical MRI experiments were performed using 9.4T Agilent instrument located at University of Illinois at Chicago (UIC)’s Research Resource Center (RRC) facility. Briefly, T2 weighted images were acquired using fsems sequence with TE= 36 ms, TR= 3000 ms, FOV = 36 mm, matrix size =128x128, slice thickness = 1 mm, # of averages = 4, total experiment time = 54 sec.
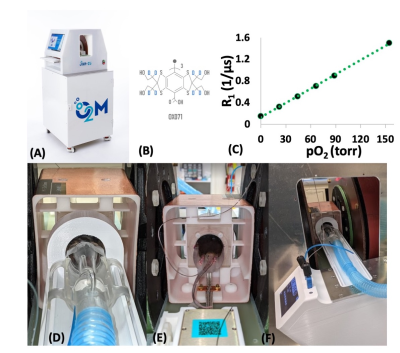
EPROI: Oxygen imaging experiments were performed using O2M’s small animal oxygen imager, JIVA-25TM (Figure 1A). JIVA-25TM provides three-dimensional pO2 maps with high spatial, temporal, and pO2 resolution for objects up to 40 mm. For reporting oxygen concentration, JIVA-25TM uses injectable trityl radicals OX071 (Figure 1B) with relaxation rates R1 linearly related to the absolute pO2 (Figure 1C). For the in vivo measurements, animals are placed in a horizontal resonator suitable for in vivo imaging and connected to an animal life support system. A 32 mm diameter by 35 mm length resonator (Figure 1D-1F) was specifically built for the whole-body mouse in vivo oxygen imaging. In vivo oxygen imaging using EPROI requires the infusion of trityl via intravenous (IV) or intraperitoneal (IP) route. We used pulse EPR inversion recovery methodology for pO2 image acquisition (3). 654 equal solid angle projections were acquired with maximum gradient of 15 mT/m and an isotropic field of view of 4.24 cm. Images were reconstructed using filtered back projection algorithm. Images were acquired in 10 minutes and had 0.66 mm spatial and ~1 torr pO2 resolution. MRI images were registered with oxygen images using a custom built Matlab program to locate the devices in the animals.
Result and Discussion
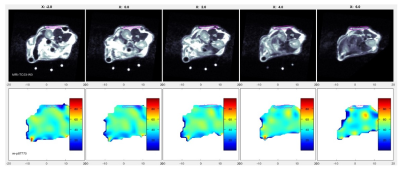
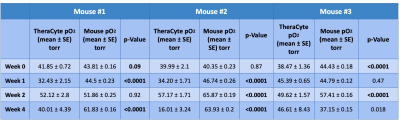
Figure 2 shows example pO2 maps along with registered MRI of a cell loaded TheraCyte device in an animal. Table 1 shows 4 weeks of pO2 statistics for the devices implanted in the animals. In most cases, the device pO2 were lower compared to the overall mouse pO2. In all cases, the device pO2 were significantly lower at week 4 compared to the initial week 0 pO2. Further histological analysis is needed to see the cause of lower pO2 in devices.Conclusion
This study aims at establishing the whole-body pO2 imaging technology of implanted islet encapsulation devices in mice model. We successfully performed 4-week study of cell-loaded TheraCyte devices implanted in mice and show that the device pO2 were significantly different than overall mouse pO2 in all cases. Further research is needed how to increase pO2 in devices that can keep cells healthy and functional during the time of implantation. We expect that these measurements will be helpful to scientists developing cell encapsulation devices and guide them how to improve device performance. Whole body mouse oxygen imaging of implanted devices is a novel development in the field of EPR imaging and we will discuss technical challenges and path forward in the presentation.Acknowledgements
We acknowledge the support of NIH R44 CA224840, NSF 2028829, and JDRF 3-SRA-2020-883-M-B grants to O2M Technologies, LLC (PI: Kotecha). We also acknowledge the support of our collaborators: Dr. Cherie Stabler (University of Florida), Dr. Minglin Ma (Cornell University), Dr. Klearchos Papas (University of Arizona), and Dr. Howard Halpern (University of Chicago).References
1. Bowers et al., Acta Biomater. 95:131-151, 2019.
2. Coronel et al., Biomaterials, 129:139-151, 2017.
3. Fernandez et al., Front. Bioeng. Biotechnol. Conference abstract:10th world biomaterial congress 2016.
4. https://theracyte.com.
5. Wang et al., Sci. Adv. Eabd5835, 2021.
6. Wang et al., Nature Comm., 12:5846, 2021.
7. Epel et al., J. Mag Reson. 280:149-157, 2017.
8. Kotecha et al., Tissue Eng Part C Methods, 24(1):14-19, 2018.
Figures