3421
Feasibility and repeatability of oxygen-enhanced T1 measurements in primary colorectal cancer: a prospective study in 22 patients.
Davide Prezzi1, Radhouene Neji1,2, Isabel Dregely1, Sami Jeljeli1, Paul Bassett3, Gary Cook1, and Vicky Goh1
1King's College London, London, United Kingdom, 2Siemens Healthineers, Frimley, United Kingdom, 3Statsconsultancy Ltd, Amersham, United Kingdom
1King's College London, London, United Kingdom, 2Siemens Healthineers, Frimley, United Kingdom, 3Statsconsultancy Ltd, Amersham, United Kingdom
Synopsis
Oxygen-enhanced ΔT1 tumor measurements were feasible in 12/22 colorectal cancer patients by means of single-slice MOLLI. A small but statistically significant negative tumor ΔT1 was found overall, in line with the expected response in well perfused and oxygenated tissue [mean (95% CI) ΔT1 = -33 (-41, -25) msec; mean tumor T1 = 1545 ± 163 msec]. A significant negative MOLLI ΔT1 was found in muscle. Repeatability and interobserver agreement were acceptable. Multi-slice variable-flip-angle VIBE tumor T1 measurements were degraded by motion or susceptibility artefact. Technical developments aimed at mitigating artefact and amplifying ΔT1 are needed for future clinical translation.
INTRODUCTION
Tumor hypoxia is an important adverse prognostic factor, inducing adaptive changes in cancer cells that make them more resistant to radiation and chemotherapy1. Imaging biomarkers that identify tumor hypoxia are needed for prognostication, therapeutic triage and development of new hypoxia-targeting therapies2.Changes in the longitudinal relaxation time (ΔT1) induced by respiratory oxygen challenge have shown promise as biomarkers of tissue oxygenation in healthy volunteers3, preclinical cancer models4 and small cancer patient cohorts5-7.We aimed:
- To assess the feasibility of tumor T1 measurements with oxygen-enhanced MRI (OE-MRI) at 3T in primary colorectal cancer (CRC).
- To measure the within-subject repeatability of OE-MRI tumor T1 measurements.
- To test for significant ΔT1 in tumor and non-tumor tissue (muscle and fat).
- To measure the interobserver agreement of OE-MRI tumor T1 measurements.
METHODS
Following ethical approval and informed consent, adults with histologically-proven CRC and a tumor ≥2 cm were recruited from a single tertiary center. Exclusions were standard contraindications to contrast MRI, prior treatment for CRC and chronic obstructive pulmonary disease.Participants underwent OE-MRI at 3T (Biograph mMR, Siemens), breathing room air followed by pure oxygen at a rate of 15 L/min for 15 min. Anonymised DICOM images were analyzed off-line in a custom-built environment (Matlab 2019b; MathWorks®).
Two T1-mapping methods were employed. Single-slice axial data was acquired at mid-tumor level using a single breath-hold modified Look-Locker inversion recovery (MOLLI) sequence. Multi-slice axial data covering the entire tumor was acquired using a breath-hold 3D variable-flip-angle spoiled gradient echo sequence (VFA-VIBE), corrected for B1 field inhomogeneity [Figure 1].
Six repeated MOLLI and six VFA-VIBE sequences were acquired sequentially before and 3 min after the start of oxygen.
Two radiologists scored the sequences from each dataset for motion and susceptibility artefacts, using a subjective 4-point scale. Sequences affected by moderate or severe artefact were excluded from analysis. Regions-of-interest (ROI) were drawn around the tumor and over fat and muscle on the first acquired map from each sequence, and copied onto subsequent maps.
A statistician utilized three-level and two-level statistical models for overall and per-patient comparison of tumor signal values, respectively. Standard deviation (SD) of measurements within patients was used to examine repeatability. Inter-observer agreement was assessed using the Bland-Altman limits of agreement.
RESULTS
Twenty-two participants were recruited (14 men, 8 women, median age: 59 years; median BMI: 27.1). Tumors had a mean (SD) diameter of 5.7±1.3 cm and were primarily located in the sigmoid colon (n=10) and rectum (n=8). Pure oxygen was well tolerated by all participants.In all cases, multi-slice T1 measurements (VFA-VIBE) were significantly degraded by motion and/or susceptibility artefact [Figure 2].
Single-slice MOLLI T1 measurements were of sufficient quality in 12/22 tumors. Mean tumor ROI size was 1,629 ± 594 voxels. Mean tumor T1 was 1545 ± 163 msec. Overall mean (95% CI) ΔT1 was -33 (-41, -25) msec for observer 1, and –29 (-40, -19) msec for observer 2 (P<0.001). Per-patient analysis yielded statistically significant negative ΔT1 in 8 out of 12 cases (66%) [Figure 3].
MOLLI T1 SD was 14 msec for observer 1 and 17-20 msec for observer 2, suggesting acceptable repeatability when compared to mean ΔT1.
No significant MOLLI ΔT1 was found in fat (P = 0.35). A significant negative MOLLI ΔT1 was found in muscle (P <0.001), in line with the expected response in healthy perfused tissue3.
Mean (SD) MOLLI T1 difference between observers was -5 (68) msec, suggesting acceptable agreement on average. Bland-Altman 95% limits of agreement were -138 to 128 msec.
DISCUSSION
Motion and susceptibility artefact were major obstacles to successful T1 measurements in the colon-rectum.Multi-slice T1 maps were of insufficient quality.T1 was successfully measured in 12/22 tumors by means of single-slice MOLLI. A highly statistically significant overall negative ΔT1 was found, in line with the expected response in well perfused and oxygenated tissue, where hemoglobin molecules are oxygen-saturated and free dioxygen plasma molecules shorten the longitudinal relaxation time. Concurrently acquired contrast MRI confirmed positive enhancement in all tumor voxels.
Significant positive MOLLI ΔT1 was found in 3 cases (25%); in all 3 cases, matching positive ΔT1 was found in muscle, suggesting likely interference of motion between measurements.
MOLLI ΔT1 were also small in magnitude, even though statistically significant, corresponding to a decrease of approximately 2% from mean tumor T1.
MOLLI T1 measurement repeatability and interobserver agreement were on average acceptable, but measurement SD and inter-observer differenced were of the same order of magnitude as mean ΔT1.
CONCLUSION
Sequence developments mitigating motion and susceptibility artefacts are needed to successfully measure oxygen-induced ΔT1 in primary colorectal cancer.A small but significant oxygen-induced negative ΔT1 was demonstrated in colorectal tumors using MOLLI, corresponding to the expected change in normoxic tissue. Technical developments aimed at amplifying ΔT1 magnitude are needed for successful clinical translation.
Acknowledgements
King’s College London and UCL Comprehensive Cancer Imaging Centre is funded by the CRUK and EPSRC in association with the MRC and DoH (England). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This work was additionally supported by the Wellcome/EPSRC Centre for Medical Engineering at King’s College London [WT 203148/Z/16/Z].References
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38-47.
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393-410.
- O'Connor JP, Naish JH, Jackson A, Waterton JC, Watson Y, Cheung S, et al. Comparison of normal tissue R1 and R*2 modulation by oxygen and carbogen. Magn Reson Med. 2009;61(1):75-83.
- O'Connor JP, Boult JK, Jamin Y, Babur M, Finegan KG, Williams KJ, et al. Oxygen-Enhanced MRI Accurately Identifies, Quantifies, and Maps Tumor Hypoxia in Preclinical Cancer Models. Cancer Res. 2016;76(4):787-95.
- Little RA, Jamin Y, Boult JKR, Naish JH, Watson Y, Cheung S, et al. Mapping Hypoxia in Renal Carcinoma with Oxygen-enhanced MRI: Comparison with Intrinsic Susceptibility MRI and Pathology. Radiology. 2018;288(3):739-47.
- Salem A, Little RA, Latif A, Featherstone AK, Babur M, Peset I, et al. Oxygen-enhanced MRI Is Feasible, Repeatable, and Detects Radiotherapy-induced Change in Hypoxia in Xenograft Models and in Patients with Non-small Cell Lung Cancer. Clin Cancer Res. 2019;25(13):3818-29.
- Bane O, Besa C, Wagner M, Oesingmann N, Zhu H, Fiel MI, et al. Feasibility and reproducibility of BOLD and TOLD measurements in the liver with oxygen and carbogen gas challenge in healthy volunteers and patients with hepatocellular carcinoma. J Magn Reson Imaging. 2016;43(4):866-76.
Figures
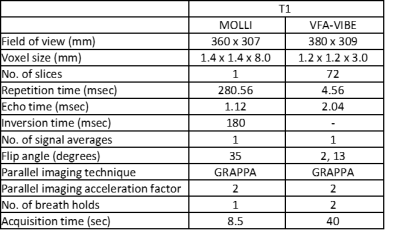
Figure 1. Sequence acquisition parameters.
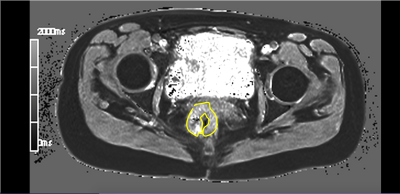
Figure 2. Mid rectal tumor. VFA-VIBE T1 mid-tumor slice. Signal within the tumor, contoured in yellow, is degraded by susceptibility artefact from rectal gas and luminal contents.
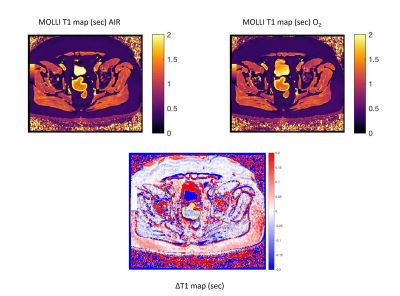
Figure 3. Examples
of successful MOLLI acquisition and subtraction ΔT1 map in a sigmoid colon tumor, contoured in
yellow.
DOI: https://doi.org/10.58530/2022/3421