3416
Quantitative Susceptibility Mapping for Gold Fiducial Marker Localization in Prostate for MRI-Only Radiation Therapy Treatment Planning
Jie Deng1, Chenyang Shen1, Junjie Wu1, and Steve B Jiang1
1Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, United States
1Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
MRI-only simulation permits superior soft tissue contrast for tumor tissue characterization and target margin delineation. One bottleneck preventing MRI-only simulation to be widely applied in prostate cancer EBRT is the tedious marker localization process on MR images as compared to that on a CT. The fiducial and calcification on MRI also appear to be similar, which reduces the confidence level of the clinician in fiducial localization, and further increases the time needed. In this study, we developed a framework using quantitative susceptibility mapping to automatically identify the fiducial markers based on phase information of gradient-echo MRI.
INTRODUCTION
As the most widely used imaging modality in treatment simulation and planning for prostate cancer external beam radiation therapy (EBRT), CT has been employed in routine clinical workflow to identify the fiducial markers in prostate at the planning stage, which serves as the reference for target localization and tracking during treatment using the on-board KV imaging function of a medical linear accelerator (LINAC). Compared to CT simulation, recent advancement in MRI-only simulation permits superior soft tissue contrast for tumor tissue characterization and target margin delineation. One bottleneck preventing MRI-only simulation to be widely applied in prostate cancer EBRT is the tedious marker localization process on MR images as compared to that on a CT. A clinician must manually navigate through all the slices of an MRI to identify the signal voids caused by fiducial. Such a process is time consuming while it requires the clinician to have expertise in both MRI and prostate cancer EBRT. The fiducial and calcification on MRI also appear to be similar, which reduces the confidence level of the clinician in fiducial localization, and further increases the time needed. In this study, we developed a framework using quantitative susceptibility mapping (QSM) to automatically identify the fiducial markers based on phase information of gradient-echo MRI. QSM exploits the magnetic susceptibility as the source of contrast between implanted gold seeds and soft tissue background or other tissues [1].METHODS
13 prostate cancer patients who underwent both MRI and CT simulation were involved in this study. Each patient has three gold fiducial markers (1.2x3.0 mm) implanted before the simulation. The MRI simulation was performed on a 1.5T scanner with a 3D 6-echo gradient echo (GRE) sequence (TR=28ms, TE = 3.1-24.6ms with ΔTE=4.3ms, flip angle=15°, FOV=180×180mm2, voxel size = 0.5×0.5×2mm3, scan time=3 mins) while the CT simulation was performed with standard prostate protocol (120 kV, 1×1×2 mm3). The proposed framework extracts magnitude and phase of GRE images, on which the fiducial markers were localized based on QSM and recorded in binary structure files. MRI QSM was generated within manually drawn prostate contours by the following steps: (1) nonlinear fitting of the complex images to generate the total field map (ΔBNFfitTol), (2) Laplacian phase unwrapping, (3) background field removal using projection onto dipole field (PDF) method to generate local field map (ΔBNFfitLoc), and (4) morphology enabled ΔBNFfitLoc-to-susceptibility (χ) inversion with background zero referencing (MEDI+0), where a binary mask within each prostate contour is generated by thresholding the magnitude image to assign 0 to pixels with signal voids and 1 to background. A binary seed mask and a calcification mask were generated by thresholding the QSM. The two QSM masks were imported back to the treatment planning system (TPS) and overlaid with MRI, which can then be utilized as the reference to guide target localization during treatment. For validation purpose, we registered the MR images along with the identified fiducial markers to the CT images based on the patient anatomy. The localization accuracy was evaluated by comparing the QSM generated masks against the fiducial markers on CT. To benchmark the performance of the proposed QSM framework, we compared the localization accuracy with that of manual localization from an experienced medical physicist.RESULTS
A summary of the identification accuracy can be found in Table 1. QSM generated distinctive contrast for calcifications and gold fiducial markers. Among the 13 patients, the proposed QSM framework was able to accurately localize all the fiducial markers on 11 of them. For the other two cases, the proposed algorithms missed two markers (one for each patient). On the other hand, the medical physicist was able to successfully segment fiducial markers accurately for 10 out of the 13 patients. Note that it took on average ~8 mins for the physicist to identify all three seeds in one patient. The time needed is expected to increase for a clinician with less experience. Fig. 1 shows a representative patient in which all 3 seeds were well identified by both QSM and the physicist, moreover, the QSM differentiated the seeds from the calcification. Fig. 2 shows a patient with bilateral hip implants in which the physicist failed to identify any of the seeds due to poor MR image quality with the presence of many signal voids in prostate whereas the QSM method distinguished all 3 seeds.DISCUSSION
Previous work reported the use of QSM for brachytherapy seeds, CT-markers, and different types of fiducial markers visualization [2] and investigated the feasibility of QSM-based fiducial marker identification and synthetic CT generation towards MRI-only treatment planning for prostate EBRT [3]. The evaluation study demonstrated that the proposed QSM framework was able to achieve comparable performance compared to the experienced medical physicist, and substantially improve efficiency of fiducial localization in prostate MRI. It has been seamlessly integrated into the TPS system and ready for clinical testing in MRI-only radiotherapy treatment planning.CONCLUSION
We implemented the clinical workflow of utilizing QSM to automatically identify gold seed fiducials in prostate MRI. It helps to improve the efficiency and increase the confidence level of the clinician in the fiducial marker localization during MRI guided radiation therapy.Acknowledgements
No acknowledgement found.References
[1] Ruetten PPR, Gillard JH, Graves MJ. Introduction to Quantitative Susceptibility Mapping and Susceptibility Weighted Imaging. Br J Radiol. 2019 Sep;92(1101):20181016. doi: 10.1259/bjr.20181016. Epub 2019 Jul 26. PMID: 30933548; PMCID: PMC6732929.[2] Nosrati R, Paudel M, Ravi A, Pejovic-Milic A, Morton G, Stanisz GJ. Potential applications of the quantitative susceptibility mapping (QSM) in MR-guided radiation therapy. Phys Med Biol. 2019 Jul 16;64(14):145013. doi: 10.1088/1361-6560/ab2623. PMID: 31151120.[3] Nosrati R, Lam WW, Paudel M, Pejović-Milić A, Morton G, Stanisz GJ. Feasibility of using a single MRI acquisition for fiducial marker localization and synthetic CT generation towards MRI-only prostate radiation therapy treatment planning. Biomed Phys Eng Express. 2021 Jun 2;7(4). doi: 10.1088/2057-1976/ac0501. PMID: 34034242.Figures
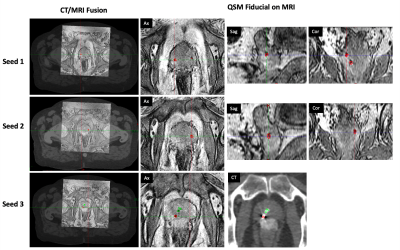
Figure 1. In a representative patient, all 3 seeds were well identified by both QSM and the medical physicist, moreover, the QSM differentiated the seeds from the calcification. CT was used as the reference of the locations of the fiducial markers.
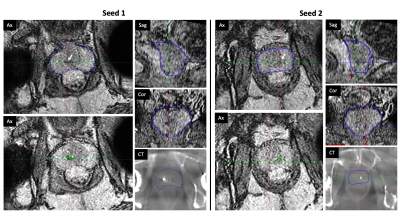
Figure 2. In a patient with bilateral hip implants in which the physicist failed to identify any of the seeds due to poor MR image quality with the presence of many signal voids in prostate, whereas the QSM method distinguished all 3 seeds. CT was used as the reference of the locations of the fiducial markers.
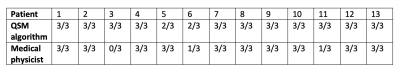
Table 1. Accuracy of fiducial localization using QSM algorithm and manual identification by the experienced medical physicist. The first number in each cell indicates the number of fiducial markers that were accurately localized, and the second number indicates the total actual number of fiducial markers in each patient.
DOI: https://doi.org/10.58530/2022/3416