3410
Characterizing Restricted Diffusion in Pre-/Post- treatment Gliomas Using Time-dependent Diffusion MRI at Ultra-high-gradient Human 3.0T1Biology and Applied Physics, GE Research, Niskayuna, NY, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Walter Reed National Military Medical Center, Bethesda, MD, United States, 4GE Healthcare, Stockholm, Sweden
Synopsis
Time-dependent diffusion MRI has shown high specificity to non-Gaussian restricted diffusion, which improves assessing cytoarchitectural alternations in pre-/post- treatment glioma. However, the characterization of time-dependent diffusivity ADC(f) and kurtosis MK(f) at short diffusion time using oscillating gradient spin echo (OGSE) diffusion encoding is limited. In our preliminary studies at ultra-high-gradient 3.0T, larger changes of ADC(f) and MK(f) were shown in glioblastoma compared to a low-grade astrocytoma, indicating highly restricted microenvironment as confirmed on histopathology. OGSE-based time-dependent diffusion showed promise for evaluating cytoarchitectural alternations in gliomas. ADC(f) and MK(f) of contrast-enhancing lesions in three post-glioblastoma-treatment patients showed strong time-dependence.
INTRODUCTION
Non-invasive imaging-based characterization of tumor grades in pre-biopsy/treatment glioma and differentiation of pseudoprogression from glioblastoma recurrence in post-treatment contrast-enhancing lesions are important for patient counseling and surgical/treatment planning. Diffusion weighted imaging (DWI) and diffusion kurtosis imaging (DKI), which are sensitive to water mobility1 and non-Gaussian diffusion2, respectively, have been used to characterize cytoarchitectural alteration in high grade gliomas and recurrent glioblastoma3. However, the assessment of cytoarchitectures using diffusion measurements can be confounded by other factors4 in the presence of tumor heterogeneity. Time-dependent diffusion MRI (dMRI) has shown high specificity to non-Gaussian restricted diffusion of the water in tumor cells5, which improves the characterization of specific tissue microstructure6,7. Oscillating gradient spin echo (OGSE) diffusion encoding imaging achieves a short diffusion time and has shown improved sensitivity to probe tissue microstructure6,8. The characterization of OGSE-based time-dependent diffusion in glioma patients, however, is limited by gradient performance of clinical whole-body systems (40-80 mT/m). We proposed to characterize time-dependent diffusivity and kurtosis in glioma patients using OGSE dMRI at ultra-high-gradient human 3.0T with gradient performance 3-5X that of clinical whole-body MRI.METHODS
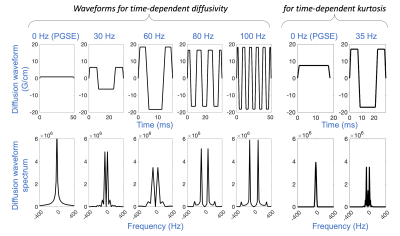
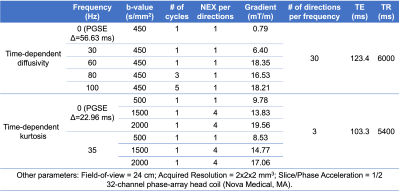
Data Acquisition: Five glioma patients were studied under an IRB-approved protocol. Two biopsy-naïve patients with suspected glioma were scanned in a 3.0T MRI system (MR750, GE Healthcare, WI) modified with a head-only MAGNUS9 gradient coil (200 mT/m amplitude and 500 T/m/s slew rate). Clinical routine preoperative and postoperative neuroimaging were also performed before and after surgical biopsy. Three patients undergoing treatment for glioblastoma showing contrast-enhancing lesions on clinical follow-up MRI were scanned with the 3.0T MAGNUS MRI system. In each patient’s exam, two OGSE dMRI acquisitions were obtained: 1) five frequencies ranging from 0 Hz to 100 Hz at b-value of 450 s/mm2; and 2) two frequencies of 0 Hz and 35 Hz at b-values of 0, 500, 1500, 2000 s/mm2. Diffusion waveforms and imaging parameters are shown in Figure 1 and Table 1. T1-weighted MPRAGE anatomical structural images and T2-weighted PROPELLER images were also acquired.Image reconstruction: All diffusion images were corrected for geometric distortion and registered to T1-weighted anatomical images. Apparent diffusivity coefficient at each frequency ADC(f) of 0-100 Hz and mean kurtosis MK(f) of 0 Hz and 35 Hz were calculated from the two OGSE diffusion MRI, after correction for diffusion gradient nonlinearity10.
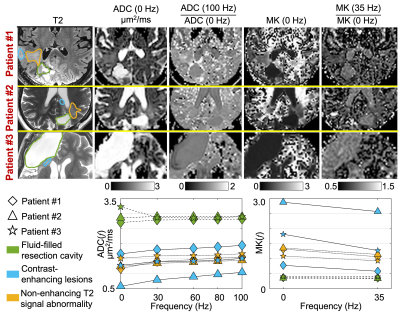
Data analysis: In the diffusion images of the pre-biopsy/treatment patients, regions-of-interest (ROIs) in the biopsied regions of the suspected tumors were drawn using preoperative and postoperative neuroimages as reference. In the diffusion images of the post-glioblastoma-treatment patients, contrast-enhancing lesions (using clinical MRI as reference), non-enhancing T2 signal abnormality, and fluid-filled resection cavity were segmented.
RESULTS
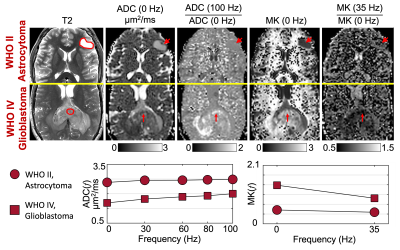
A diffuse astrocytoma, IDH-mutant, WHO grade II and a glioblastoma, IDH-wildtype, WHO grade IV were diagnosed from histopathology in the two patients who were imaged pre-biopsy/treatment (Figure 2). ADC(f) of glioblastoma started low at 0 Hz, increased with a steep slope as frequencies increased to 30 Hz, and then increased slowly as frequencies increased to 100 Hz. ADC(f) of astrocytoma showed a higher value at 0 Hz and a relatively shallow slope as frequencies increased. MK(f) of glioblastoma showed a high value at 0 Hz but decreased significantly at 30 Hz. MK(f) of astrocytoma was lower at 0 Hz and decreased slowly at 35 Hz.In all the three patients scanned post-glioblastoma-treatment (Figure 3), ADC(f) was high (~3 μm2/ms), and MK(f) was low and frequency-independent in the fluid-filled resection cavity (green). ADC(f) of contrast-enhancing regions (blue) increased significantly from 0 Hz to 30 Hz and showed a higher rate of increase in one patient (also lower ADC at 0 Hz) compared to the other two patients. ADC(f) further increased from 30 Hz to 100 Hz at a slower rate. MK(f) decreased from 0 Hz to 35 Hz in all three patients. The regions with non-enhancing T2 signal abnormality (yellow) in all three patients showed increasing ADC(f) and decreasing MK(f) as a function of frequencies. The absolute values and relative changes, especially from 0 Hz to 30 Hz, were different between patients.
DISCUSSION AND CONCLUSION
We have preliminarily characterized time-dependent diffusivity and kurtosis in glioma patients using OGSE dMRI. In the pre-biopsy/treatment patients, the strong time-dependence of ADC and MK in glioblastoma (i.e., significant difference between 0 Hz and ~30 Hz), which indicate highly restricted diffusion, was consistent with the higher cellularity as confirmed on histopathology. Compared to the conventional ADC and MK, ADC(f) and MK(f) provided specific information on restricted diffusion, showing promise for improving tumor characterization.In the post-glioblastoma-treatment patients, the low and frequency-independent MK(f) indicates Gaussian diffusion in fluid-filled resection cavity, which is consistent with the expected microscopic picture of mostly unrestricted diffusion in these regions. Compared to MK at 0 Hz, time-dependent kurtosis improves the differentiation of restricted diffusion from other diffusion processes that result in non-zero kurtosis, e.g., a mixture of Gaussian diffusion compartments. The time-dependent ADC(f) and MK(f) indicate restricted diffusion in both contrast-enhancing lesions and non-enhancing T2 signal abnormality regions. Further correlation between the MRI measurements with subsequent clinical diagnosis (pseudoprogression vs. tumor recurrence) is ongoing. Future investigation in a larger patient cohort is needed to fully characterize the diagnostic performance of OGSE dMRI.
Acknowledgements
The authors acknowledge the funding support from U.S. Department of Defense, W81XWH-16-2-0054. The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University. The authors thank Blessing Ndukwe for patient recruitment.References
1. Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y. Usefulness of diffusion‐weighted MRI with echo‐planar technique in the evaluation of cellularity in gliomas. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 1999;9(1):53-60.
2. Cauter SV, Veraart J, Sijbers J, Peeters RR, Himmelreich U, Keyzer FD, Gool SWV, Calenbergh FV, Vleeschouwer SD, Hecke WV, Sunaert S. Gliomas: Diffusion Kurtosis MR Imaging in Grading. Radiology 2012;263(2):492-501.
3. Wu X-f, Liang X, Wang X-c, Qin J-b, Zhang L, Tan Y, Zhang H. Differentiating high-grade glioma recurrence from pseudoprogression: Comparing diffusion kurtosis imaging and diffusion tensor imaging. European Journal of Radiology 2021;135:109445.
4. Tietze A, Hansen MB, Østergaard L, Jespersen SN, Sangill R, Lund TE, Geneser M, Hjelm M, Hansen B. Mean Diffusional Kurtosis in Patients with Glioma: Initial Results with a Fast Imaging Method in a Clinical Setting. American Journal of Neuroradiology 2015;36(8):1472-1478.
5. Hope TR, White NS, Kuperman J, Chao Y, Yamin G, Bartch H, Schenker-Ahmed NM, Rakow-Penner R, Bussell R, Nomura N, Kesari S, Bjørnerud A, Dale AM. Demonstration of Non-Gaussian Restricted Diffusion in Tumor Cells Using Diffusion Time-Dependent Diffusion-Weighted Magnetic Resonance Imaging Contrast. Frontiers in Oncology 2016;6(179).
6. Reynaud O. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Frontiers in Physics 2017;5(58).
7. Roberts TA, Hyare H, Agliardi G, Hipwell B, d’Esposito A, Ianus A, Breen-Norris JO, Ramasawmy R, Taylor V, Atkinson D. Noninvasive diffusion magnetic resonance imaging of brain tumour cell size for the early detection of therapeutic response. Scientific reports 2020;10(1):1-13.
8. Reynaud O, Winters KV, Hoang DM, Wadghiri YZ, Novikov DS, Kim SG. Surface‐to‐volume ratio mapping of tumor microstructure using oscillating gradient diffusion weighted imaging. Magnetic resonance in medicine 2016;76(1):237-247.
9. Foo TKF, Tan ET, Vermilyea ME, Hua Y, Fiveland EW, Piel JE, Park K, Ricci J, Thompson PS, Graziani D, Conte G, Kagan A, Bai Y, Vasil C, Tarasek M, Yeo DTB, Snell F, Lee D, Dean A, DeMarco JK, Shih RY, Hood MN, Chae H, Ho VB. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magnetic Resonance in Medicine 2020;n/a(n/a).
10. Tan ET, Marinelli L, Slavens ZW, King KF, Hardy CJ. Improved correction for gradient nonlinearity effects in diffusion‐weighted imaging. Journal of Magnetic Resonance Imaging 2013;38(2):448-453.
Figures