3401
Improving cortical bone imaging using a novel Flexible Ultra Short Echo time (FUSE) sequence for optimal synthetic CT generation1Division of Biomedical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 2Department of Mechanical Engineering, University of Saskatchewan, Saskatoon, SK, Canada, 3Research Collaboration Manager, Siemens Healthcare Limited, Oakville, ON, Canada, 4Department of Radiology, University of Manitoba, Winnipeg, MB, Canada
Synopsis
Ultrashort echo time (UTE) pulse sequences are used in synthetic computed tomography (sCT) generation for MR-based radiation treatment planning (RTP). This study used a novel Flexible UTE sequence (FUSE) for improving cortical bone imaging. Using a human skull, we applied the optimized UTE acquisition techniques and parameters and verified the improvements in overall image quality of cortical bone in comparison to a product UTE sequence. Also, we have shown the feasibility and potential of our FUSE sequence for generating the sCT data that is clinically equivalent to the traditional CT for RTP.
Introduction
There is growing demand to perform MRI-only radiation treatment planning (RTP)1–3. In routine RTP, computed tomography (CT) images are used for contour delineations and dose calculations. Synthetic computed tomography (sCT) is an MRI-derived image that tries to closely mimic an actual CT image for radiation treatment simulation and planning4. One critical challenge of all sCT generation methods is the difficulty in obtaining adequate cortical bone signal in conventional MR images due to the cortical bone’s quick relaxation times. Ultrashort echo time (UTE) sequences use specialized acquisition techniques to efficiently shorten the time-of-echo (TE) to acquire signals quickly so that the cortical bone can be directly imaged5,6. Given that the quality of the UTE images of cortical bone is paramount in RTP, the acquisition techniques (e.g., trajectories) and parameters must be carefully selected. Therefore, this study aims to 1) verify and optimize the use of a novel in-house Flexible Ultra Short Echo time (FUSE) sequence for improved cortical bone imaging in terms of artifact reduction and contrast delineation and 2) to use the FUSE sequence to generate sCT data for creating a radiation treatment plan and assessing its dosimetric equivalence to a plan created using a traditional CT image.Methods
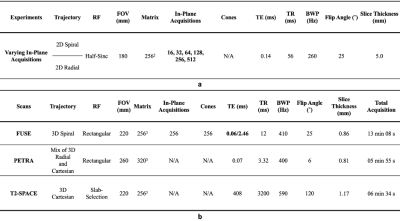
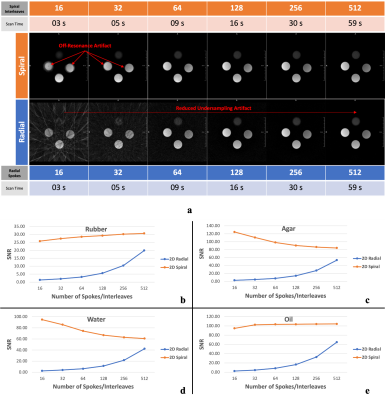
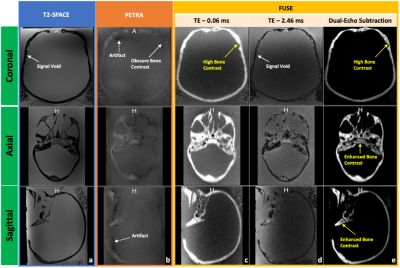
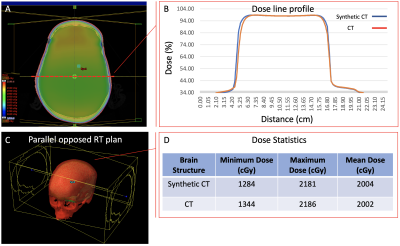
An in-house phantom containing both a short-T2 component (rubber) and long-T2 components (water, oil, and agar) and a human skull (dried cadaver) were scanned on a 3 Tesla MR system (MAGNETOM PrismaFit, Siemens Healthineers, Erlangen, Germany) using a novel in-house UTE sequence, FUSE. The FUSE sequence (IDEA, VE11C, Siemens Healthineers, Erlangen, Germany) provides user-configurable acquisition options, for example, radial and spiral trajectories with an adaptable combination of in-plane and out-of-plane k-space trajectory lines. The phantom was used to determine the optimal selections of the trajectory type and the number of k-space in-plane trajectory lines by considering the appearance of unwanted artifacts, Signal-to-Noise Ratio (SNR), and scan time (Figure 1. a)7. The dry human skull was fully immersed in a saline solution (1 litre H2O, 3.6 grams NaCl8) for scanning. The protocol included two FUSE scans (TEs: 0.06 and 2.46 ms), a product UTE scan (Pointwise Encoding Time reduction with Radial Acquisition, PETRA9), and a T2-weighted scan (SPACE) (Figure 1. b). The skull was also scanned on a Siemens CT scanner (SOMATOM Definition Edge, Siemens Healthineers, Erlangen, Germany). The scan used a head-first supine position with 120 kVp, 210 mA, 200 mm as field-of-view (FOV), 0.6 mm as slice thickness, 512×512×264 as the reconstructed matrix, and 0.392 mm2 as the in-plane resolution. For sCT generation, we imported the FUSE-derived difference image into the radiation treatment planning system (Eclipse Version 13.6, Varian Medical Systems, Pal Alto, USA) and applied a bulk density assignment to the contoured bony anatomy using a nominal value of 1196 HU (mass density 1.85 g/cm3). Using the FUSE-derived sCT data, we created a simple two-field 6 MV parallel opposed radiation treatment plan using dose fractionation of 2000 cGy in five fractions. For comparison, the same plan was generated using the CT image of the same human skull. For both plans, minimum, maximum, and mean dose metrics were calculated and compared.Results
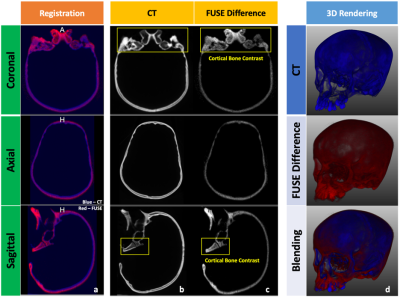
Overall, the spiral trajectory provided better controlled under-sampling of off-resonance artifacts and higher SNR than the radial trajectory when the same number of in-plane trajectory lines (e.g., 64-512) were used (Figure 2). With the optimized scan parameters (spiral trajectory with 256 spiral interleaves), the FUSE sequence used a scan with a TE of 0.06 ms to provide a well-defined delineation and enhanced contrast of the cortical bone using the dual-echo subtraction method (Figure 3). The FUSE sequence was able to delineate the human skull’s morphology, and the result was comparable to CT (Figure 4). The treatment plans using the FUSE-derived sCT and the traditional CT are clinically identical with the mean dose less than 1% difference and agreeable dose profiles (Figure 5).Discussion
The spiral trajectory of our FUSE sequence provides demonstrable higher SNR and better artifact reduction than the radial trajectory. The optimal use of our FUSE sequence is essential for the bone imaging that is used for synthetic CT generation. To our knowledge, this has not been extensively studied until now, due to the lack of user-configurable UTE sequences like FUSE. Many scan parameters of the PETRA sequence are not configurable and inherently restrict its use, while our FUSE sequence provides more flexibility on the parameter’s optimization, e.g., changes of the Spiral-Cones trajectory’s in-plane spiral interleaves or out-of-plane cones, for a higher bone contrast without artifacts. The CT scan of the skull bone verified the feasibility of the FUSE sequence for providing comparable bone contrast10. Last, our study shows it is feasible to use the FUSE sequence to generate sCT data for the skull bone and apply the sCT data in RTP to produce equivalent dosimetry to the authentic CT data: the difference of mean dose between the two plans is less than 1%. This minor dosimetrical difference suggests the FUSE-based synthetic CT data is clinically equivalent to traditional CT.Conclusion
In summary, we have shown the feasibility and potential of our FUSE sequence for generating the sCT data that is clinically equivalent to the traditional CT for RTP.Acknowledgements
The authors acknowledge our funding sources, Mitacs-Accelerate IT10545, IT19172 and Arthritis Society Young Investigator Operating Grant. The authors also thank Siemens Healthineers for their technical supports.References
1. Hoffmann A, Oborn B, Moteabbed M, Yan S, Bortfeld T, Knopf A, et al. MR-guided proton therapy: a review and a preview. Radiat Oncol Lond Engl. 2020 May 29;15:129.
2. Chandarana H, Wang H, Tijssen RHN, Das IJ. Emerging Role of MRI in Radiation Therapy. J Magn Reson Imaging JMRI. 2018 Dec;48(6):1468–78.
3. Owrangi AM, Greer PB, Glide-Hurst CK. MRI-only treatment planning: benefits and challenges. Phys Med Biol. 2018 Feb 26;63(5):05TR01.
4. Stanescu T, Jans H-S, Pervez N, Stavrev P, Fallone BG. A study on the magnetic resonance imaging (MRI)-based radiation treatment planning of intracranial lesions. Phys Med Biol. 2008 Jul 7;53(13):3579–93.
5. Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: Projection reconstruction MR imaging. Radiology. 1991;
6. Gold GE, Pauly JM, Macovski A, Herfkens RJ. MR Spectroscopic imaging of collagen: Tendons and knee menisci. Magn Reson Med. 1995;34(5):647–54.
7. Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging JMRI. 2007 Aug;26(2):375–85.
8. Jackson E, Bronskill M, Drost D, Och J, Pooley R, Sobol W, et al. Acceptance Testing and Quality Assurance Procedures for Magnetic Resonance Imaging Facilities [Internet]. AAPM; 2010 Dec;
9. Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med. 2012;67(2):510–8.
10. Soliman AS, Burns L, Owrangi A, Lee Y, Song WY, Stanisz G, et al. A realistic phantom for validating MRI‐based synthetic CT images of the human skull. Med Phys [Internet]. 2017;
Figures