3395
Deep Learning Based Automatic Pipeline for Quantitative Assessment of Thigh Muscle Fatty Infiltration in Post-traumatic Osteoarthritis
Sibaji Gaj1, Brendan L. Eck1, Dongxing Xie1, Richard Lartey1, Charlotte Lo1, Mingrui Yang1, Kunio Nakamura1, Carl S. Winalski2, Kurt Spindler3, and Xiaojuan Li1
1Biomedical Engineering, Cleveland Clinic, Cleveland, OH, United States, 2Radiology, Cleveland Clinic, Cleveland, OH, United States, 3Orthopaedics, Cleveland Clinic, Cleveland, OH, United States
1Biomedical Engineering, Cleveland Clinic, Cleveland, OH, United States, 2Radiology, Cleveland Clinic, Cleveland, OH, United States, 3Orthopaedics, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Quantitative assessment of thigh muscle morphology and fatty infiltration (FF%) in post-traumatic osteoarthritis is limited. In this work, we developed a deep learning based accurate segmentation method for muscles, bone and adipose tissue from thigh MRI and used these segmentation for automated quantification of FF and cross sectional area(CSA) of these tissues. 16 patients at 10 years after ACL reconstruction were studied. The proposed method showed significant improvement in segmentation metrics (Dice, Average surface distance (ASD)) and CSA compared with popular U-Net based deep learning models. For CSA and FF% quantification, automated methods had similar measurements compared with manual segmentation.
Introduction:
Patients after anterior cruciate ligament injury (ACL) have a high risk of developing PTOA despite ACL reconstruction (ACLR). Thigh muscle (including quadriceps and hamstrings) weakness, atrophy, and impaired neuromuscular functions following ACLR have been well documented and are suggested as potential risk factors for PTOA development after ACLR1,6. However, studies on quantitative assessment of thigh muscle morphology and fatty infiltration are limited7-9. One hurdle is the need for accurate segmentation of muscle and manual and semi-automated segmentation are time consuming and prone to intra- and inter-operator variations. Previous studies showed promising results for fully automated segmentation using U-Net models but the non-discriminative MRI texture around muscle boundary limits these model’s performance in small dataset8-9. The Aim of the study was to develop a novel deep learning segmentation method for fast and accurate automatic thigh muscle segmentation and area and fat fraction quantification from MRI.Method:
16 patients (age 35.25±5.49, BMI 25.5±4.28, 10 Male) were scanned using a 3T MR scanner and anterior/posterior receive arrays for bi-latera thigh imaging at 10 years after ACLR. The MRI protocol contains high-resolution T1-weighted turbo spin-echo (TSE, TR=607-795ms, TE=10ms, Matrix Size= (432-512)x(400-432)x28)) and 6-point Dixon MRI scans (TSE, TR=16.37ms, TE=1.23ms, Matrix Size= 256x(192-256)x28)) . Dixon MRI scans were processed using a vendor-independent algorithm to obtain quantitative maps of fat fraction (FF%). Manual segmentation of three muscle groups (Quadriceps, Hamstrings, and Other muscle (adductor group, gracilis and sartorius)) was performed using the TSE scans after co-registration to the Dixon MRI scans via intensity-based rigid registration. Other tissue labels (Subcutaneous Fat, Inner Bone, and Inner Bone Marrow) were obtained using post processing on manual muscle segmentation and TSE scans. A modified encoder-decoder based convolution neural network (CNN) with dense connections and atrous spatial pyramid pooling was developed for better utilizing multi-context information learned at various depth of deep network and aggregating the multi-scale information in segmentation10,11. Two popular CNN models (2D-UNet and 3D-UNet) were implemented and evaluated 12. While proposed and 2D-UNet used single slices as input and produced segmentation mask for that slice, 3D-UNet used consecutive 8 slices as input and produced corresponding segmentation masks. We used 4-fold cross validation for training and testing (3:1 ratio for training and testing) of these models. Multiclass dice coefficient loss along with Adam optimizer were used for all model training. We assessed the model’s segmentation accuracy using dice score (0 indicates no overlap, and 1 is perfect) and ASD in mm. After segmentation, the mean FF% and CSA for each muscle group was obtained from 4 image slices located at the mid-thigh. The difference between manual and automated segmentation based FF% (∆FF%) along with correlation were used for evaluation. For comparison of CSA quantification, the percentage of absolute mean difference with manual segmentation (∆CSA %) and the correlation were used.Results:
Fig1 shows the segmentation performance for different methods. As our dataset had relatively few scans to train, 3D-UNet performance was not optimal and failed to segment where discriminative textures were not present within the muscle groups (Fig1,C). The improvement of the proposed method in terms of Dice and ASD as given in Fig 2. It had average dice of 0.977 and ASD of 0.28. The ASD improvement were 28.45% and 66% compared with 2D-UNet and 3D-UNet. The proposed method had highest improvement in Hamstrings of the operated side (24.5%) and in other muscles of the contralateral side (29.43%) within the muscle labels (Please note that muscle labels were only segmented fully manually while others labels were semi-auto). In Fig 3, the correlation and ∆CSA % were provided for the quantitative assessments. The proposed method’s performance was better compared to U-Net models within the muscle groups. It had lowest ∆CSA% of 0.98% for Quadriceps of the contralateral side and highest ∆CSA % of 6.01% in Hamstring of the operated side. Similarly, the ∆FF% and correlation were provided for FF% quantification in Fig 4. The average ∆FF% were 0.16 for proposed segmentation method which was lower than the previously reported scan-rescan variability of ~1% with manual segmentation. The ∆FF% was highest in Hamstrings for both proposed and U-Net based automatic segmentation methods.Conclusion and Discussion:
We developed a fully automatic accurate intramuscular fat fraction quantification pipeline for thigh muscle using a small multisite and multivendor dataset. The model had outperformed the U-Net based segmentation models in terms of segmentation metrics. Such pipelines will greatly facilitate quantitative muscle assessment in large-scale cohort studies or trials, and facilitate clinical translation of quantitative muscle assessment to clinical practice. The largest difference in FF% between our proposed method and manual segmentation was observed in Hamstrings of the operated side (0.51%). It was primarily caused by 3 patients who showed extreme fat replacement of semitendinosus within Hamstrings, and the model classified those area as fat instead of muscle (one example in Fig 4). Nonetheless, the overall FF% difference of 0.51% is still much lower than the scan-rescan variability (1%). The performance can be improved further by including such cases with high fatty infiltration in training in future studies.Acknowledgements
No acknowledgement found.References
[1] Brown, Thomas D., et al. "Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease." Journal of orthopaedic trauma 20.10 (2006): 739-744. [2] Thomas, Abbey C., et al. "Epidemiology of posttraumatic osteoarthritis." Journal of athletic training 52.6 (2017): 491-496. [3] Pedroso, Maria Gabriela, et al. "Fatty infiltration in the thigh muscles in knee osteoarthritis: a systematic review and meta-analysis." Rheumatology international 39.4 (2019): 627-635. [4] Palmieri-Smith, Riann M., and Abbey C. Thomas. "A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury." Exercise and sport sciences reviews 37.3 (2009): 147-153. [5] Tourville, Timothy W., et al. "Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction." The American journal of sports medicine 42.2 (2014): 302-311. [6] Petersen, Wolf, et al. "Return to play following ACL reconstruction: a systematic review about strength deficits." Archives of orthopaedic and trauma surgery 134.10 (2014): 1417-1428. [7] Kemnitz, Jana, et al. "Clinical evaluation of fully automated thigh muscle and adipose tissue segmentation using a U-Net deep learning architecture in context of osteoarthritic knee pain." Magnetic Resonance Materials in Physics, Biology and Medicine 33.4 (2020): 483-493. [8] Chen, Yongsheng, et al. "Automation of Quantifying Axonal Loss in Patients with Peripheral Neuropathies through Deep Learning Derived Muscle Fat Fraction." Journal of Magnetic Resonance Imaging 53.5 (2021): 1539-1549. [9] Ding, Jie, et al. "Deep learning-based thigh muscle segmentation for reproducible fat fraction quantification using fat–water decomposition MRI." Insights into imaging 11.1 (2020): 1-11. [10] Huang, Gao, et al. "Densely connected convolutional networks." Proceedings of the IEEE conference on computer vision and pattern recognition. 2017. [11] Chen, Liang-Chieh, et al. "Encoder-decoder with atrous separable convolution for semantic image segmentation." Proceedings of the European conference on computer vision (ECCV). 2018.[12] Ronneberger, Olaf, Philipp Fischer, and Thomas Brox. "U-net: Convolutional networks for biomedical image segmentation." International Conference on Medical image computing and computer-assisted intervention. Springer, Cham, 2015.Figures
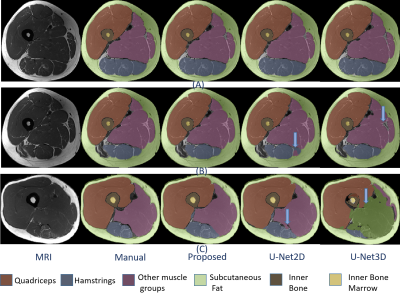
Figure 1: Overlay of segmentations from
manual and automatic methods. Rows presents different slices and columns are
segmentation provided by different methods. The discrepancy between manual and
auto segmentation is pointed by arrow.
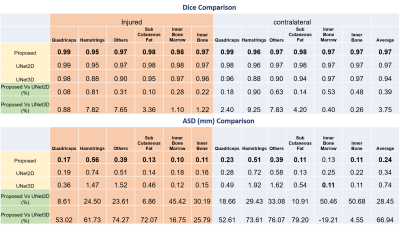
Figure 2: Dice comparison of the proposed
method with U-Net based segmentation methods. Best performance is marked in
bold.
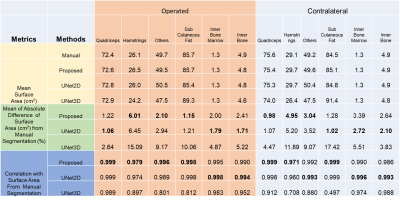
Figure 3: Comparison of surface area
between auto and manual segmentation methods. The best performances have been
marked bold.
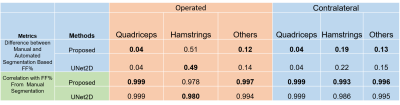
Figure 4: Comparison of FF% between auto
and manual segmentation methods. The best performances have been marked
bold.

Figure 5: FF map overlay using manual and
automatic segmentation. The fatty infiltration (marked by arrow) had replaced
muscle fully which is causing error in final quantification.
DOI: https://doi.org/10.58530/2022/3395