3391
Reproducibility of Alkaline Inorganic Phosphate Quantification using 31P-Magnetic Resonance Spectroscopy at 3T1Kinesiology, University of Massachusetts at Amherst, Amherst, MA, United States, 2Center for Health and Human Performance, Institute for Applied Life Sciences, University of Massachusetts at Amherst, Amherst, MA, United States, 3Neuroscience, Biomedicine, and Movement Science, University of Verona, Verona, Italy, 4Centre de Resonance Magnetique Biologique et Medicale, Marseille, France, 5Human Magnetic Resonance Center, Institute for Applied Life Sciences, University of Massachusetts at Amherst, Amherst, MA, United States, 6Institute for Applied Life Sciences, University of Massachusetts at Amherst, Amherst, MA, United States
Synopsis
Alkaline inorganic phosphate (Pialk) is a promising biomarker of mitochondrial content in vivo that is technically challenging to assess at 3T. The absolute and relative reproducibility of Pialk in the quadriceps muscle across two visits were assessed in healthy sedentary-to-moderately active young adults using 31P-MRS at 3T. The results of this study demonstrate an excellent absolute and relative reproducibility of the quantification of Pialk in the quadriceps muscle at 3T provided that the coil is positioned at the mid-thigh.
Introduction
The field of phosphorus magnetic resonance spectroscopy (31P-MRS) has seen a renewed interest for an alkaline Pi (Pialk) resonance, given its potential as a marker of mitochondrial content 1–10. However, several technical barriers [e.g., low concentration, small chemical shift between Pi cytosolic (Picyt) and Pialk] have hampered its broad application to clinical populations. To date, a robust and reproducible method to quantify Pialk in the skeletal muscle using 31P-MRS with a 3T whole-body MR system has yet to be established. This is an important first step toward the translation of this potential surrogate marker of mitochondrial content into clinical practice. Therefore, the goal of this project was to quantify the reproducibility (i.e., within-subject reproducibility along with measurement and processing errors) of Pialk measured at 3T by a surface coil in the skeletal muscle using 31P-MRS in sedentary-to-moderately active young adults.Methods
Resting 31P-MRS of the quadriceps muscles was acquired, on two separate visits, from 13 healthy, sedentary-to-moderately active young adults (23±1 yrs. old; males n = 7; females n = 6) using a whole-body 3T Siemens Skyra MR system. Measurement variability related to coil position, shimming procedure, and spectral analysis were also quantified. 31P-MRS data were acquired with a 31P-1H dual-tuned surface coil positioned on the right quadriceps using a pulse-acquire sequence with the following parameters: TR = 1500 ms, Bandwidth: 4000 Hz, 2048 data points, Nominal Flip Angle: 80°, 200 Averages, 1H decoupling WALTZ-4 at 50% i.e., 240 ms, hard pulse 100 ms). The carrier frequency was placed between the PCr and Pi peak. All measurements were performed within SAR limits. Spectra were processed with atime-domain fitting routine using the Advanced Method for Accurate, Robust and Efficient Spectral (AMARES) fitting algorithm incorporated into the CSIAPO software11. Test-retest absolute and relative reproducibility from repeat scans were analyzed using the coefficient of variation (CV) and intra-class correlation coefficients (ICC), respectively.Results
All the phosphorus metabolites (i.e., six prominent peaks corresponding to the three phosphate groups of adenosine triphosphate (ATP), phosphocreatine (PCr), the phosphodiesters (PDE) glycerophosphoethanolamine (GPE) and glycerophosphocholine (GPC), and Pi) were detected in the quadriceps with noticeable separation of Pialk and cytosolic Pi (Picyt). Figure 1 illustrates a representative 31P spectrum acquired in the quadriceps muscle at 3T. Both Pialk (CV: 10.6 ± 5.4%; ICC: 0.80, Figure 2) and the ratio Pialk/Picyt (CV: 10.3 ± 8.1%; ICC: 0.71, Figure 2) demonstrated excellent within-subject reproducibility. Differences in proximo-distal coil placement along the length of the quadriceps affected Pialkquantification (CV: 21.1%) due to impaired field homogeneity in the region closer to the knee (Figure 3). In contrast, measurement variability on consecutive scans from the same muscle sample with repeated advanced shimming procedure (CV: 6.6%) and from the automated spectral processing procedure were small in the present experimental set-up (CV: 2.3%).Discussion & Conclusion
Albeit two-fold greater than other well-resolved metabolites such as PCr and PDE (data not shown), test-retest variability of Pialk was still very low, and several metrics indicated excellent relative and absolute reliability of the quantification of Pialk in the quadriceps at 3T. Coil location significantly affected Pialk quantification, with higher [Pialk] in the distal region of the thigh due to magnetic field inhomogeneity. With the coil positioned mid-thigh, consecutive scans within the same day with repeat of the shimming procedure (Full Width Half Maximum of PCr <12.5 Hz) resulted in small Pialkvariability (CV=6.6%). The automated spectral processing procedure was also highly reproducible for Pialk (CV ≤ 2.3%). Collectively, the results of the present study support the reproducibility of Pialk quantification by 31P MRS at 3T using a surface coil positioned mid-thigh on the quadriceps, and provide a strong foundation for the validation of Pialk as a surrogate marker of mitochondrial content.Acknowledgements
The authors wish to thank the participants for volunteering for this study. This work was funded in part by the NIH National Heart, Lung, and Blood Institute (R00 HL125756-03).References
1. Cohen, S. M. et al. 31P Nuclear Magnetic Resonance Studies of Isolated Rat Liver Cells. Nature 273, 554–556 (1978).
2. Ogawa, S. et al. High-Resolution 31P Nuclear Magnetic Resonance Study of Rat Liver Mitochondria. Proceeding of the National Academy of Sciences of the United States of America 75, 1796–1800 (1978).
3. Garlick, P. B., Soboll, S. & Bullock, G. R. Evidence that Mitochondrial Phosphate is Visible in 31P NMR Spectra of Isolated, Perfused Rat Hearts. NMR in Biomedicine 5, 29–36 (1992).
4. Garlick, P. B., Brown, T. R., Sullivan, R. H. & Ugurbil, K. Observation of a Second Phosphate Pool in the Perfused Heart by 31P NMR; is this the Mitochondrial Phosphate? Journal of Molecular and Cellular Cardiology 15, 855–858 (1983).
5. Dufour, S. et al. Temperature Dependence of NMR Relaxation Times of Nucleoside Triphosphates and Inorganic Phosphate in the Isolated Perfused Rat Liver. Effect on Pi Compartmentation. Journal of Magnetic Resonance, Series B113, 125–135 (1996).
6. Thiaudiere, E., Gallis, J.-L., Dufour, S., Rousse, N. & Canioni, P. Compartmentation of Inorganic Phosphate in Perfused Rat Liver: Can Cytosol be Distinguished from Mitochondria by 31P NMR? Federation of European Biochemical Societies 330, 231–235 (1993).
7. van Oorschot, J. W. M. et al. 31P MR Spectroscopy and Computational Modeling Identify a Direct Relation between Pi Content of an Alkaline Compartment in Resting Muscle and Phosphocreatine Resynthesis Kinetics in Active Muscle in Humans. PLoS ONE 8, 1–9 (2013).
8. Kan, H. E. et al. In vivo 31P MRS Detection of an Alkaline Inorganic Phosphate Pool with Short T1 in Human Resting Skeletal Muscle. NMR in Biomedicine 23, 995–1000 (2010).
9. Klepochová, R. et al. Differences in muscle metabolism between triathletes and normally active volunteers investigated using multinuclear magnetic resonance spectroscopy at 7T. Frontiers in Physiology 9, (2018).
10. Valkovič, L. et al. Skeletal Muscle Alkaline Pi Pool is Decreased in Overweight-to-Obese Sedentary Subjects and Relates to Mitochondrial Capacity and Phosphodiester Content. Scientific Reports 6, 1–9 (2016).
11. le Fur, Y. et al. Grid-free Interactive and Automated Data Processing for MR Chemical Shift Imaging Data. Magnetic Resonance Materials in Physics, Biology and Medicine 23, 23–30 (2010).
Figures
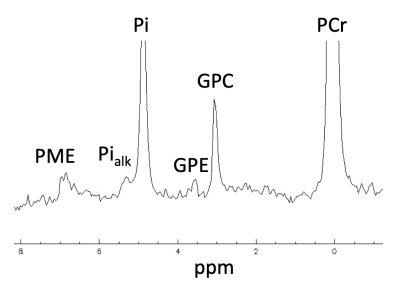
Figure 1. Representative example of a 31P-MRS spectra in the quadriceps muscle of a healthy young adult (22 ± 3 yrs. old) at 3T, with the region between 0 and 8 ppm enlarged. A 2Hz Gaussian filtered was used for apodization. PCr, phosphocreatine; GPC, glycero-3-phosphocholine; GPE, glycero-3-phosphoethanolamine, Pi, cytosolic inorganic phosphate; Pialk, alkaline inorganic phosphate, PME, phosphomonoesters.
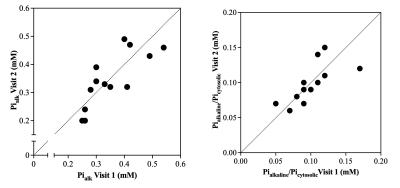
Figure 2. Scatter plot of [Pialk] (left panel) and the ratio of Pialkaline and Picytosolic (right panel) comparing visit 1 and visit 2 (average: 4 days between visits, range: 2-7 days). Dashed lines represent the ‘line of identity’.
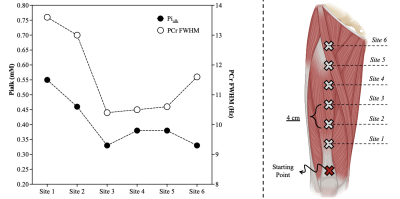
Figure 3. [Pialk] across the six different sites (left). Six different ‘sample volumes’ were measured along the longitudinal length of the quadriceps (n=1), in 4 cm intervals, starting at the patella and with the muscle fully relaxed (right). FWHM: Full Width Half Maximum of PCr.