3381
Comparison of Spiral and Cartesian Acquisitions for Rapid Hyperpolarized 129Xe Ventilation Mapping in Pediatric Cystic Fibrosis Lung Disease1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Division of Respiratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
A rapid 3D stack-of-spirals (3D-SoS) sequence was used for hyperpolarized 129Xe ventilation imaging in pediatric cystic fibrosis and healthy controls, allowing for 5x reduction in scan duration and breath-hold compared to a commonly used 2D gradient echo (2D-GRE) sequence. The ventilation defect percent (VDP) measured with 3D-SoS was not significantly different from VDP measured with 2D-GRE. Additionally, the transient drop in SpO2 associated with xenon breath-holds was reduced with the shorter breath-hold afforded by 3D-SoS. In future, the reduced acquisitions durations offered by 3D-SoS may allow for improved tolerability, or may be traded for improved resolutions.
Introduction
MRI with hyperpolarized (HP) 129Xe gas is a promising approach for imaging pediatric cystic fibrosis (CF). Previous work has shown HP 129Xe MRI to be safe, reproducible, and sensitive to early disease and treatment1–4. Xenon in the airspaces is typically evaluated with slice-selective 2D gradient recalled echo (2D-GRE) acquisitions yielding measures of ventilation defect percent (VDP). Though widely adopted for 129Xe MRI, 2D-GRE scans typically require breath-hold durations of 8-12 seconds5, and usually no longer than 16 seconds, due to the Cartesian nature of k-space acquisition. Such breath-holds may be increasingly difficult for subjects to perform, especially for very sick and/or younger patients.Non-Cartesian image acquisitions such as spirals acquire k-space data more efficiently per excitation, reducing overall scan duration, presenting a faster alternative to standard GRE imaging6. More recently, spiral-based acquisitions have enabled dynamic 129Xe ventilation acquisitions7,8 or 3D isotropic imaging9. Nevertheless, these methods generally remain underutilized for this application. Previously, our group demonstrated preliminary 129Xe ventilation data using a fast 3D stack-of-spirals (3D-SoS) acquisition compared to typical 2D-GRE. However, this was limited to a small number of healthy adult participants10.
In this study we present a similar HP 129Xe imaging comparison using the same 3D-SoS sequence in a group of healthy control and stable CF pediatric participants.
Methods
Thirteen pediatric participants were imaged with informed consent under institutional and Health Canada approval. Of these, 5 were healthy controls (2M, 3F, ages=13.8±2.2 years) and 8 were CF participants (3M, 5F, ages=15.4±1.6 years). Imaging was performed on a clinical 3T MRI system (Siemens Prisma, Erlangen, Germany). HP 129Xe MRI was performed with a flexible transmit/receive vest coil (Clinical MR Solutions, Brookfield, WI). 129Xe was polarized to 28.5±2.7% (9820, Polarean, Durham, NC). Both naturally abundant and isotopically enriched (~85% 129Xe) sources of xenon were used. Xenon was dosed to 10% of total lung capacity (TLC) calculated based on height and sex11, then balanced with N2 bringing the total inhaled volume to 1/6 TLC. 2D-GRE imaging was performed with acquisition parameters adherent to guidelines recently suggested by the Xenon MRI Clinical Trials Consortium (XeMRI-CTC)5. The acquisition time ranged from 6.2-8.8 seconds depending on the number of slices required (10-14 slices). Previously described 3D-SoS imaging10 was performed in the same participants with acquisition times ranging from 1.2-1.8 seconds. Detailed imaging parameters for both sequences are found in Table 1. 2D-GRE and 3D-SoS datasets were acquired with individual breath-holds of xenon. The transient SpO2 drop (lowest value within two minutes post-inhalation) was expressed as a percent change relative to the SpO2 immediately after inhalation.3D-SoS images were reconstructed using a non-uniform FFT12, and both 2D-GRE and 3D-SoS were interpolated to a resolution of 2×2×15mm3. 129Xe images were bias corrected and registered to the segmented 1H thoracic cavity mask. Defect regions were identified as voxels with signal intensity less than 60% of the whole-lung mean11. Linear regression and Bland-Altman analysis were used to compare VDP measures between acquisitions.
Results
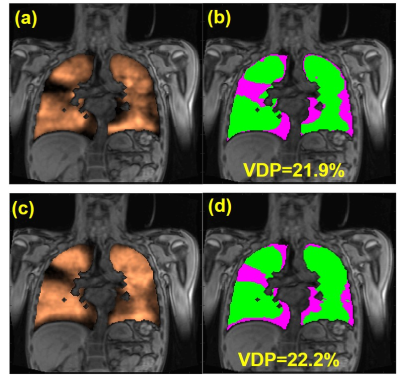
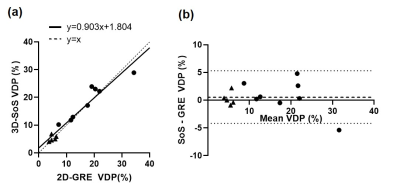
Representative ventilation images for both acquisitions are shown for a healthy and CF participant (Figures 1 and 2, respectively). Figure 3 shows VDP analysis and defect cluster mapping for a representative CF participant for both acquisitions. 2D-GRE and 3D-SoS VDPs were 5.02±1.06% and 5.28±1.08% respectively for healthy participants, and 18.05±8.26% and 18.75±6.74% respectively for CF participants. The VDPs measured with 2D-GRE and 3D-SoS were not significantly different in either the healthy or CF groups (p>0.05). Both 2D-GRE and 3D-SoS VDPs were able to differentiate between healthy and CF participants (p<0.01 and p<0.001 respectively). Linear regression of 3D-SoS VDP against 2D-GRE VDP is shown in Figure 4a. Bland-Altman analysis of these VDP measures is shown in Figure 4b. The percent change in SpO2 was reduced (p<0.05) between 2D-GRE and 3D-SoS breath-holds (−5.2±3.5% and −2.1±2.7% respectively).Discussion
In this work we present an alternative 3D-SoS approach to typical 2D-GRE acquisitions for HP 129Xe MRI in pediatric participants that is approximately 5× faster. The transient SpO2 drop associated with xenon inhalation was reduced with the shorter 3D-SoS breath-hold, suggesting improved tolerability. Ventilation defect patterns were observed to be very similar between acquisitions (Figure 3). Quantitative comparison of VDP analysis between 2D-GRE and 3D-SoS shows good agreement between the two approaches. Linear regression indicates 3D-SoS VDP is highly linearly correlated with 2D-GRE VDP. Bland-Altman analysis shows a low bias between measures (<1%) and a reasonably low variability. Most of the variability observed was related to registration of 129Xe data to 1H thoracic cavity masks, likely due to each dataset requiring separate breath-holds. Improvements to the image registration, or the acquisition of 129Xe/1H data within the same breath-hold (leveraging the speed of non-Cartesian approaches) should help mitigate this source of variability in the future. Nevertheless, these results are comparable to previously reported intrascan reproducibility in a similar population (Bias=-0.04, 95% Limits of Agreement=[-3.10, 3.03])3.Conclusion
In conclusion, VDP values measured with 3D-SoS were acquired faster and were not significantly different from those measured with 2D-GRE, indicating potential for more widespread adoption for 129Xe MRI. The reductions in scan duration affords the flexibility to prioritize speed/tolerability (as is the case here), trade-off for improved resolution, or perform multiple imaging acquisitions in the same breath-hold.Acknowledgements
The authors thank Jacky Au, Sharon Braganza, Daniel Li, Leslie Burns, Tammy Rayner, and Ruth Weiss for assistance with imaging experiments. This work was supported by CIHR project PJT-1530399 and the Cystic Fibrosis Foundation.References
1. Walkup LL, Thomen RP, Akinyi TG, et al. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol. 2016;46(12):1651-1662. doi:10.1007/s00247-016-3672-1
2. Kanhere N, Couch MJ, Kowalik K, et al. Correlation of Lung Clearance Index with Hyperpolarized 129 Xe Magnetic Resonance Imaging in Pediatric Subjects with Cystic Fibrosis. Am J Respir Crit Care Med. 2017;196(8):1073-1075. doi:10.1164/rccm.201611-2228LE
3. Santyr G, Kanhere N, Morgado F, Rayment JH, Ratjen F, Couch MJ. Hyperpolarized Gas Magnetic Resonance Imaging of Pediatric Cystic Fibrosis Lung Disease. Acad Radiol. 2019;26(3):344-354. doi:10.1016/j.acra.2018.04.024
4. Rayment JH, Couch MJ, Mcdonald N, et al. Hyperpolarised 129 Xe magnetic resonance imaging to monitor treatment response in children with cystic fibrosis. Eur Respir J. 2019;53:1802188. doi:10.1183/13993003.02188-2018
5. Niedbalski PJ, Hall CS, Castro M, et al. Protocols for multi-site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med. 2021;86(6):2966-2986. doi:10.1002/mrm.28985
6. Salerno M, Altes TA, Brookeman JR, De Lange EE, Mugler JP. Dynamic spiral MRI of pulmonary gas flow using hyperpolarized 3HE: Preliminary studies in healthy and diseased lungs. Magn Reson Med. 2001;46(4):667-677. doi:10.1002/mrm.1244
7. Doganay O, Matin TN, Mcintyre A, et al. Fast dynamic ventilation MRI of hyperpolarized 129 Xe using spiral imaging. Magn Reson Med. 2018;79(5):2597-2606. doi:10.1002/mrm.26912
8. Chen M, Doganay O, Matin T, et al. Delayed ventilation assessment using fast dynamic hyperpolarised Xenon-129 magnetic resonance imaging. Eur Radiol. 2019:E-Pub ahead of print.
9. Willmering MM, Niedbalski PJ, Wang H, et al. Improved pulmonary 129Xe ventilation imaging via 3D-spiral UTE MRI. Magn Reson Med. 2020;84(1):312-320. doi:10.1002/mrm.28114
10. Zanette B, Friedlander Y, Munidasa S, Santyr G. Comparison of 3D Stack-of-Spirals and 2D Gradient Echo for Ventilation Mapping using Hyperpolarized 129Xe. Proc Intl Soc Mag Reson Med. 2020;28:0449.
11. Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2017;16(2):275-282. doi:10.1016/j.jcf.2016.07.008
12. Fessler JA, Member S, Sutton BP. Nonuniform Fast Fourier Transforms Using Min-Max Interpolation. IEEE Trans Signal Process. 2003;51(2):560-574.
Figures
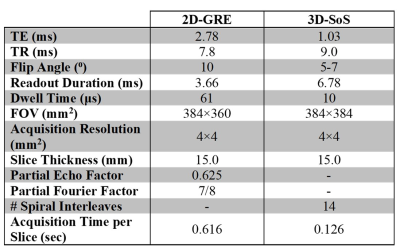
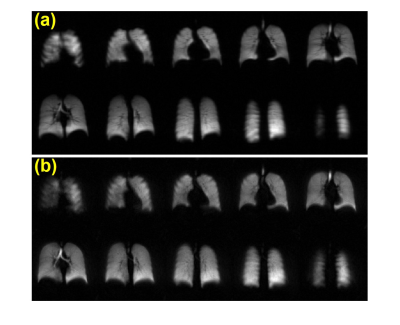
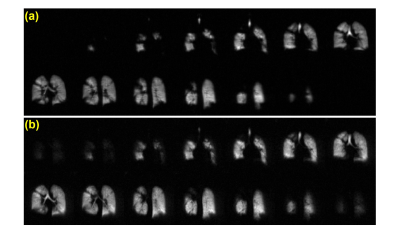
Figure 1: Representative HP 129Xe ventilation images from a healthy pediatric control participant (12 y.o. male) acquired with (a) 2D-GRE and (b) 3D-SoS.