3374
Regional Lung Ventilation Mapping at 0.55T based on Feature Tracking1Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Children's Hospital of Los Angeles, Los Angeles, CA, United States, 3Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States
Synopsis
Assessment of regional lung ventilation has significant clinical value for the diagnosis and follow-up of pulmonary diseases. High-performance 0.55T systems have provided new opportunities for pulmonary imaging due to prolonged T2* and reduced susceptibility. Here, we describe an image-based regional lung ventilation assessment method based on real-time bSSFP imaging with 0.3s temporal and 1.64x1.64 mm2 spatial resolution, and feature tracking. In healthy adult volunteers, we demonstrate the ability to detect posture-related left-right differences in ventilation, and test-retest repeatability. We detected -5% to 64% regional volume changes throughout the respiratory cycle from end of exhalation to total lung capacity.
Introduction
Functional lung imaging is of great importance for the diagnosis and evaluation of lung diseases (e.g., chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis). Conventional methods often include inhaled hypopolarized gas (1) or 100% oxygen (2) as contrast agents. In recent years, high performance low field systems have shown great advantages for 1H lung MRI due to reduced susceptibility effects and improved vessel conspicuity (3). These allow possibilities to detect regional volume changes throughout the respiratory cycle.In this work, we demonstrate the feasibility of regional ventilation map from real-time MRI at 0.55T, without requiring contrast agents, repetition, or breath holds. We demonstrate posture-related left-right differences in ventilation within the physiologic range.
Methods
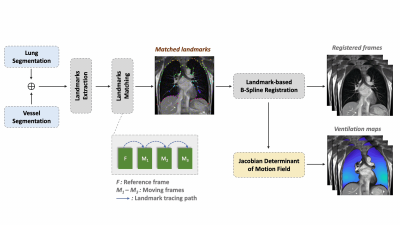
Imaging Methods: Experiments were performed using a whole body 0.55T system (prototype MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate). Data collected used a 6-channel body array (anterior) and 6 elements from a table-integrated 18-channel spine array (posterior). We used a 2D real-time balanced steady-state free precesion (bSSFP) pulse sequence with 355ms/frame temporal resolution, 1.64 x 1.64 mm2 spatial resolution, and 15mm slice thickness. Images were continuously acquired for 30s. Other parameters: coronal prescription, TE/TR = 1.67/3.70ms, flip angle = 60º, FOV = 315 x 420mm2. Two healthy volunteers (26/M, and 33/M) were scanned. Volunteer 1 was scanned twice on separate days with three breathing patterns: breathe normally (free and shallow breathing), breathe deeply with maximum inhalation and exhalation, and forced exhalation. Volunteer 2 was scanned in two positions: left side down with the right arm being stretched straight up position, and right side down with the left arm being straight up position.Feature Tracking & Analysis: Figure 1 illustrates the feature tracking pipeline. We applied a data-driven binarization algorithm (4) to lung parenchyma area and vessels, separately. Reference landmarks were extracted from the reference frame using morphological post-processing. We then performed a frame-by-frame matching between the two adjacent frames (5). After that, a landmark-based B-spline registration (6) was applied for each frame with respect to the reference frame. Jacobian determinant (JD) maps (7) were generated using the calculated deformation fields from the registration step.
Results
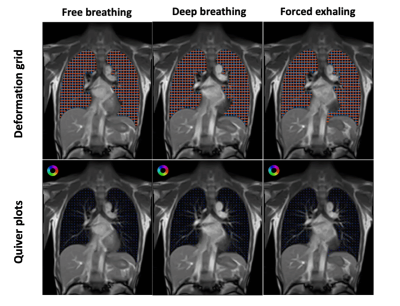
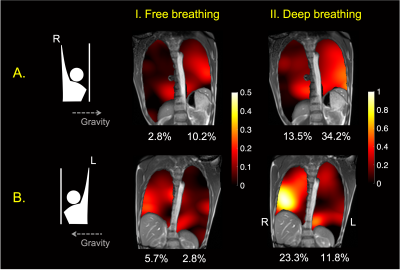
Figure 2 shows the deformation and quiver plots for different breathing maneuvers, including free breathing, deep breathing, and force exhalation. The dependent lung experiences increased ventilation relative to the other lung for all three breathing patterns. The motion and volume change for deep breathing and forced exhalation were larger, especially in the dependent lung, than the free breathing case.Figure 3 demonstrates posture-related ventilation differences. The ventilation changes in a wider range during (II.) deep breathing than (I.) free breathing. Ventilation in the left lung is higher than the right lung in the right-arm-up posture, and vice-versa, as expected. The maximum ventilation changes are generally larger in the dependent lung with smaller end-of-exhalation volume.
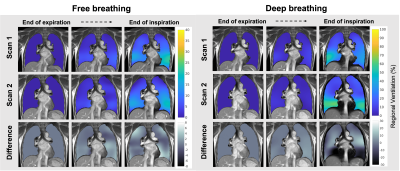
Figure 4 shows the intra-subject test-retest results. The low values in the percentage difference maps indicate the results are reproducible in different breathing maneuvers. Free breathing had a <10% volume expansion difference between the two scans, and was the most reproducible among the three respiratory patterns.
Discussion
Our posture-related experimental design revealed that real-time image acquisition can be performed and provide ventilation measurements that are physiologic. Real-time ventilation measurements will provide clinically important data in patients with abnormal lung function who previously could not be tested such as infants and patients with cognitive impairment.There are several limitations for the current 2D single slice ventilation analysis. 1) The current motion states lack synchronization between two breathing cycles, which may lead to the errors in test-retest experiments. This can be solved by temporal interpolation or performing motion-resolved reconstruction. 2) The current approach only captures 2D in-plane motion, whereas through plane motion happens in reality and can bias the deformation maps, especially in the cardiac region. 3) We assume there is no through plane volume change. Extending this work to 3D RT-MRI (8,9) could provide the absolute gas exchange values and will be our future work.
Conclusion
We demonstrate the feasibility of regional ventilation mapping from real-time MRI at 0.55T. We are able to detect posture-related left-right differences in ventilation, and good test-retest reproducibility. We are able to detect -5% to 64% regional volume changes throughout the respiratory cycle from end of exhalation to total lung capacity.Acknowledgements
We acknowledge grant support from the National Science Foundation (#1828736) and research support from Siemens Healthineers.References
1. Möller HE, Chen XJ, Saam B, et al. MRI of the lungs using hyperpolarized noble gases. Magn. Reson. Med. 2002;47:1029–1051 doi: 10.1002/mrm.10173.
2. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad P V. Noninvasive assessment of regional ventilation in the human lung using oxygen–enhanced magnetic resonance imaging. Nat. Med. 1996;2:1236–1239 doi: 10.1038/nm1196-1236.
3. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019;293:384–393 doi: 10.1148/radiol.2019190452.
4. Magoulianitis V, Han P, Yang Y, Kuo C-CJ. Unsupervised Data-Driven Nuclei Segmentation For Histology Images. arXiv preprint arXiv:2110.07147 (2021).
5. Voskrebenzev A, Gutberlet M, Kaireit TF, Wacker F, Vogel-Claussen J. Low-pass imaging of dynamic acquisitions (LIDA) with a group-oriented registration (GOREG) for proton MR imaging of lung ventilation. Magn. Reson. Med. 2017;78:1496–1505 doi: 10.1002/mrm.26526.
6. Rueckert D Hayes C, Hill DLG, Leach MO, Hawkes DJ. SLI. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 1999;18:18:712-21.
7. Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four-dimensional computed tomography: Density versus Jacobian methods. Phys. Med. Biol. 2010;55:4661–4685 doi: 10.1088/0031-9155/55/16/004.
8. Lim Y, Zhu Y, Lingala SG, Byrd D, Narayanan S, Nayak KS. 3D dynamic MRI of the vocal tract during natural speech. Magn. Reson. Med. 2019;81:1511–1520 doi: 10.1002/mrm.27570.
9. Zhao Z, Lim Y, Byrd D, Narayanan S, Nayak KS. Improved 3D real-time MRI of speech production. Magn. Reson. Med. 2021;85:3182–3195 doi: 10.1002/mrm.28651.
Figures