3371
Diffusion and perfusion MRI for prediction of HCC response to neoadjuvant immunotherapy1BioMedical Engineering and Imaging Institute (BMEII), Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Regeneron Pharmaceuticals Inc. Tarrytown, New York, NY, United States, 4Department of Pathology, Molecular and Cell Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 6The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 7Precision Immunology Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Neoadjuvant immunotherapy has the potential to decrease the risk of hepatocellular carcinoma (HCC) recurrence post resection. The objectives of this study were to assess the value of pre-treatment MRI parameters for prediction of HCC immunophenotype and response in a cohort of patients undergoing neoadjuvant immunotherapy prior to liver resection. We hypothesize that diffusion and perfusion MRI can predict HCC response to neoadjuvant immunotherapy. It was observed that abundant of TILs decreases ADC in tumors. Moreover, uptake rate is higher in highly necrotic tumors.
Purpose
Assess the value of advanced DWI and DCE-MRI measured at baseline in predicting HCC response to neoadjuvant immunotherapy.Background
HCC is the cancer with 2nd highest mortality in men worldwide1, and is the most rapidly rising cause of cancer mortality in the US2. The treatment of choice for patients with HCC is surgical resection in patients with preserved liver function. Tumor recurrence is common, with early (within 2 years) recurrence observed in approximately 50% of cases3. Immunotherapy has revolutionized cancer treatment, including HCC, with described overall response rate (ORR) to PD-1 blockade in patients with advanced HCC was 15-20%4, with the combination of anti-PD-L1 and bevacizumab with even higher ORR, at 25%5. The tumor microenvironment plays a vital role in therapy response, with previous reports suggesting higher density of tumor infiltrating lymphocytes (TILs) resulted in improved immunotherapy outcomes6. Thus, neoadjuvant immunotherapy has the potential benefit of improving outcomes. MRI provides quantitative metrics such as tissue perfusion and cellularity that can potentially be used to predict HCC response to immunotherapy.Methods
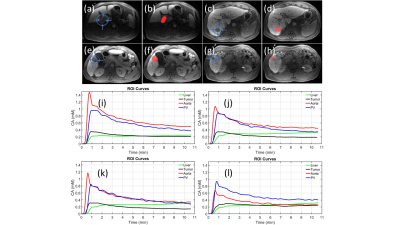
In this prospective single-center study, 17 patients (10M/7F, mean age 64±12y) underwent MRI at baseline and following completion of neoadjuvant immunotherapy (cemiplimab: anti-PD1) prior to liver resection (post treatment MRI to resection interval: 4±2 days). Multi b-value DWI was performed using SSEPI and 11 b-values (range 50-800s/mm2). DCE-MRI was performed using gadoxetate contrast. Freehand ROIs were drawn on the DCE-MRI images in the tumor, liver, aorta and portal vein (Figure 1), in tumor on the IVIM-DWI images. A dual-input dual-compartment model was used to quantify DCE-MRI parameters in tumors: arterial plasma flow Fa(ml/100ml/min), portal venous plasma flow Fp(ml/100ml/min), total plasma flow Ft(ml/100ml/min), arterial fraction ART (%), mean transit time MTT(s), interstitial volume fraction Ve (%) intracellular uptake rate ki (min-1), uptake fraction fi (%)7. Model-free parameters were also calculated: time-to-peak TTP(s) and upslope (mM/min)8. A Bayesian fitting algorithm was used to perform pixelwise mapping of pseudodiffusion coefficient (Ds), diffusion coefficient (D), and perfusion fraction (PF) in the ROIs. ADC values were extracted from a separate monoexponential fit through the signal data (b>100 s/mm2). Resected samples were assessed by a pathologist who measured degree of necrosis and the grade of tumor infiltrating lymphocytes (TILs), presence of tertiary lymphoid structure (TLS). Tumor response was assessed using RECIST 1.1 and degree of tumor necrosis at histopathology (significant tumors necrosis: STN ≥70%).Results
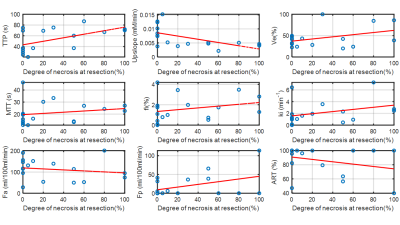
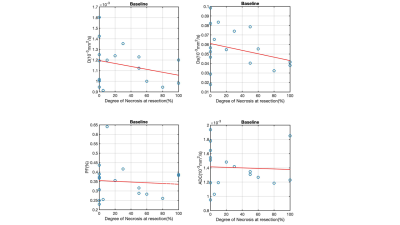
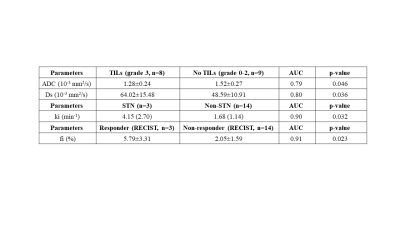
Only baseline MRI findings are discussed. 17 tumors were assessed (mean size 7.0±4.5cm). 3/17 (17.6%) patients had STN, and 3/17 (17.6%) had partial response based on RECIST. TILs were present in 12/14 tumors at histopathology. The significant results from DWI and DCE-MRI are summarized in Table 1. Tumor ki was significantly increased in patients with STN compared to nonresponders (4.15±2.70 vs 1.68±1.14, AUC=0.90, p=0.032). Uptake fraction fi was significantly higher with patients with partial response based on RECIST (responders/nonresponders 5.79±3.31/2.05±1.59, AUC=0.92, p=0.23) (Table 1). The variation of perfusion and diffusion parameters with degree of necrosis at resection are shown in Figures 2 & 3. Mean ADC was significantly lower in the patients with abundant TILS (grade 3): (1.28 ± 0.24 vs 1.52 ± 0.27, AUC=0.79, p=0.046). Kurtosis (STN/nonresponders: 13214 ±1124/7348 ± 4263, AUC=0.93, p=0.021) and skewness (STN/nonresponders: 2896 ± 256/1812 ± 776, AUC=0.90, p=0.032) of Ds were significantly higher in patients with response based on necrosis.Discussion
We observed that perfusion parameter ki (uptake rate) in HCC lesions were predictive of response of HCC to neoadjuvant immunotherapy, and uptake fraction fi (%) was significantly higher in patients with partial response (based on RECIST). In addition, tumor ADC was lower in tumors with abundant TILs. This preliminary study suggests that several MRI parameters may predict tumor immunophenotype and response to immunotherapy, which may potentially be useful in stratifying HCC patients for immunotherapy. A validation study is necessary to ensure validation of these results.Conclusion
Our initial results demonstrate the potential utility of baseline diffusion and perfusion parameters for prediction of HCC immunophenotype and for predicting response to neoadjuvant immunotherapy.Acknowledgements
This work was funded by Regeneron Pharmaceuticals Inc.References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359-86. doi: 10.1002/ijc.29210. Epub 2014 Oct 9. PMID: 25220842.
2. Siegel RL, Fedewa AS, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal Cancer Incidence Patterns in the United States, 1974-2013; JNCI J Natl Cancer Inst (2017) 109(8): djw322.
3. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015 May;261(5):947-55. doi: 10.1097/SLA.0000000000000710. PMID: 25010665.
4. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017 Jun 24;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2. Epub 2017 Apr 20. PMID: 28434648; PMCID: PMC7539326.
5. Shah NJ, Kelly WJ, Liu SV, Choquette K, Spira A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum Vaccin Immunother. 2018;14(2):269-276. doi:10.1080/21645515.2017.1403694.
6. Plesca I, Tunger A, Muller L, et al. Characteristics of Tumor-In ltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front Immunol 2020;11:364.
7. Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K. Quantification of cerebral blood flow, cerebral blood volume, and blood-brain-barrier leakage with DCE-MRI. Magn Reson Med 2009;62:205-217.
8. Hectors, SJ, Bane, O, Kennedy P, et al. Noninvasive diagnosis of portal hypertension using gadoxetate DCE-MRI of the liver and spleen. Eur Radiol 31, 4804–4812 (2021). https://doi.org/10.1007/s00330-020-07495-0.
Figures